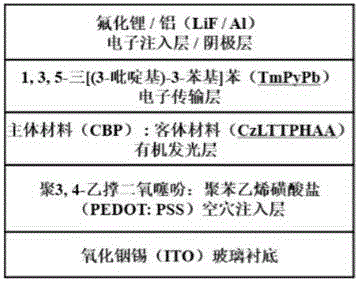
Novel fluorene bipolar fluorescent material based on anthraquinone group and application of novel fluorene bipolar fluorescent material in organic light emitting diodes
A light-emitting diode, anthraquinone group technology, applied in the field of organic electroluminescent devices, can solve the problems of unbalanced injection and transport of holes and electrons, low electron affinity, difficult electron injection, etc., and achieves high yield, The effect of good performance and great commercialization potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 12-(12,12',15,15'-tetra-n-butyl-6,6'-diisooctyl-12,15-dihydro-6H-cyclopentadiene[1,2-b: The preparation of 5,4-b'] bisfluorenyl) anthracene-9,10-dione (LTTPHAA) is as follows:
[0039]
[0040] Step 1 Preparation of intermediate 1-1: Dissolve 1.15g (1.45mmol) LTTPH in 45-75mL of chloroform, add 0.23g (1.45mmol) of liquid bromine dropwise, keep at -10-10°C, and react overnight; After extraction with methyl chloride, drying, and rotary evaporation to remove the solvent, 1 g of white solid was obtained by recrystallization from dichloromethane and ethanol, with a yield of 80%.
[0041] serial number LTTPH (g) Liquid bromine (g) Chloroform (mL) temperature(℃) product (g) 1 1.15 0.23 45 -10 0.93 2 1.15 0.23 55 0 1 3 1.15 0.23 75 10 0.96
[0042]Step 2 Preparation of LTTPHAA: 8222 Add 0.36g (0.41mmol) of intermediate 1-1 and 0.14g (0.41mmol) of 2-anthraquinone boric acid pinacol ester into a three-necked flask, va...
Embodiment 22
[0047] Example 22,2'-(12,12',15,15'-tetra-n-butyl-6,6'-diisooctyl-12,15-dihydro-6H-cyclopentadiene[1,2 -b: Preparation of 5,4-b']bifluorenyl)bis(anthracene-9,10-dione) (DAALTTPH), its synthetic route is as follows:
[0048]
[0049] Step 1 Preparation of intermediate 2-1: Dissolve 0.92g (1.16mmol) LTTPH in 45-75mL of chloroform, add 0.37g (2.32mmol) of liquid bromine dropwise, keep at -10-10°C, and react overnight; After extraction with methyl chloride, drying, and rotary evaporation to remove the solvent, 0.93 g of white solid was obtained by recrystallization from dichloromethane and ethanol, with a yield of 85%.
[0050] serial number LTTPH (g) Liquid bromine (g) Chloroform (mL) temperature(℃) product (g) 1 0.92 0.37 45 -10 0.90 2 0.92 0.37 55 0 0.93 3 0.92 0.37 75 10 0.88
[0051] Step 2 Preparation of DAALTTPH: Add 0.95g (1mmol) of intermediate 2-1 and 0.67g (2mmol) of 2-anthraquinone borate pinacol ester into a three...
Embodiment 32-
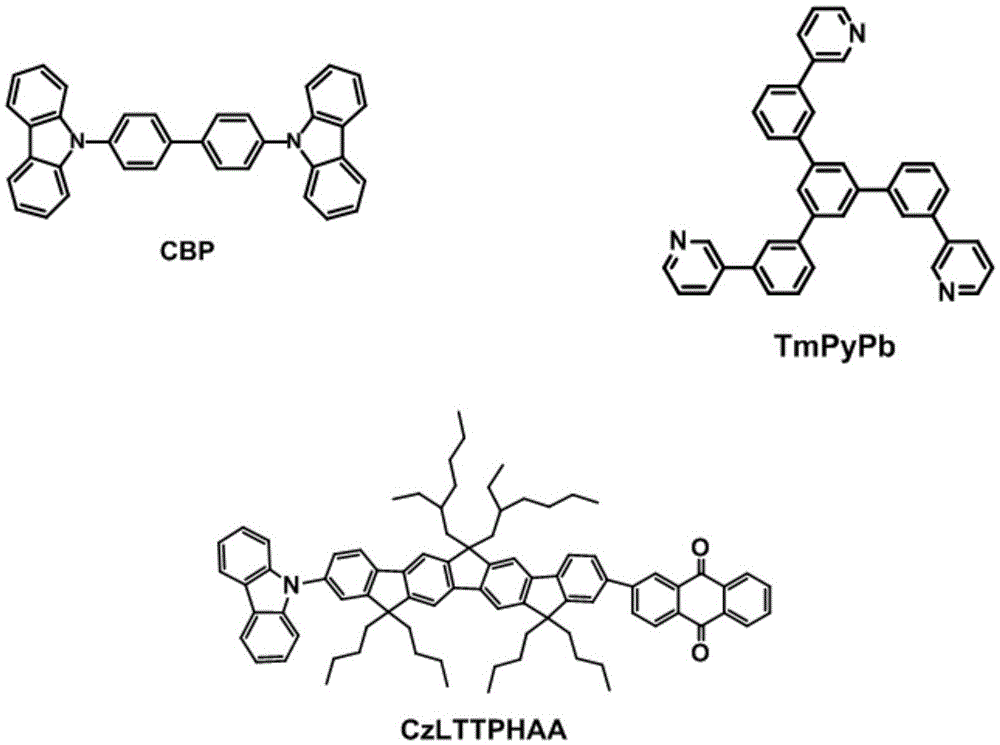
[0056] Example 32-[12,12',15,15'-tetra-n-butyl-10-(9-9H-carbazolyl)-6,6'-diisooctyl-12,15-dihydro-6H - Preparation of cyclopentadiene[1,2-b:5,4-b']difluorenyl]anthracene-9,10-dione (CzLTTPHAA), whose synthetic route is as follows:
[0057]
[0058] Step 1 Preparation of intermediate 3-1: 1.14g (1.2mmol) of intermediate 2-1 prepared in Example 2 and 0.4g (1.2mmol) 2-anthraquinoneboronic acid pinacol ester were added to a three-necked flask, Vacuumize and fill with nitrogen for 3 times, add 50mg tetrakis(triphenylphosphine)palladium; mix 5~20mL toluene / tetrahydrofuran mixed solvent (1:1) and 2~4mL potassium carbonate / potassium fluoride after deoxygenation by nitrogen bubbling Solvent (2mol / L) was added into the reaction flask respectively, heated to 80-100°C, and stirred for 24-72 hours. After the reaction was completed, it was extracted with dichloromethane, dried, and the solvent was removed by rotary evaporation, and purified by silica gel column chromatography to obtain ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com