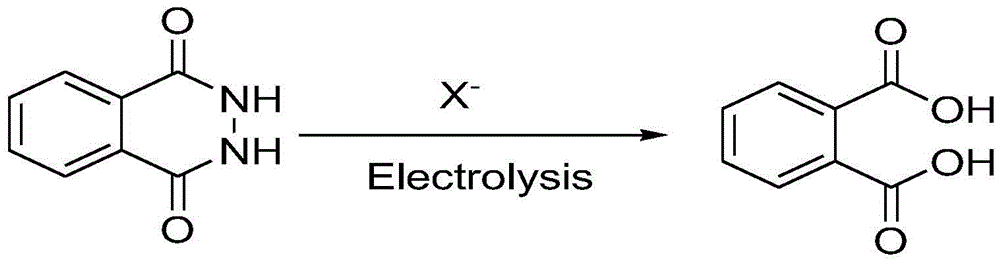Method for preparing phthalic acid through phthalylhydrazine in electrochemical oxidation manner
A technology of phthalic hydrazide and phthalic acid, which is applied in the field of preparation of phthalic acid, can solve the problems of casualties, property, large amount of addition, high price, etc., and achieve avoidance of large-scale use, mild reaction conditions, less corrosive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Phthalic acid is obtained by electrochemical oxidation of phthalic hydrazide
[0024] In the single-chamber electrolytic cell, add 50ml of water, add 6.2mg of sodium hydroxide, and adjust the pH value of the water to 10-11. Weigh phthalic hydrazide (1.0mmol) and then add sodium bromide (2mmol), use graphite sheet as anode, iron sheet as cathode, current density 5mA / cm 2 Electrolyze under constant current, and the reaction temperature is room temperature (30°C). After 5 hours and 50 minutes, the energization was completed, and the pH value of the system was adjusted to about 1 by adding hydrochloric acid, and then filtered with a Buchner funnel. Finally, heat and suspend to dry off most of the water, the heating temperature is about 80°C, and put it into the refrigerator for recrystallization. Yield: 16%.
Embodiment 2
[0025] Example 2: Phthalic acid is obtained by electrochemical oxidation of phthalic hydrazide
[0026] In the single-chamber electrolytic cell, add 50ml of water, add 6.5mg of sodium hydroxide, and adjust the pH value of the water to about 10. Weigh phthalic hydrazide (1mmol) into the system, then add sodium bromide (1mmol), use graphite sheet as anode, stainless steel sheet as cathode, current density 10mA / cm 2 Electrolyze at a constant current, and the reaction temperature is room temperature (30°C). After 4 hours, the electricity is turned on, and the pH value of the system is adjusted to about 1 by adding hydrochloric acid, and then filtered with a Buchner funnel. Finally, heat and suspend to dry off most of the water, the heating temperature is about 80°C, and put it into the refrigerator for recrystallization. Yield: 33%.
Embodiment 3
[0027] Embodiment 3: Phthalic acid is obtained by electrochemical oxidation of phthalic hydrazide
[0028] In the single-chamber electrolytic cell, add 50ml of water and 6.0mg of sodium hydroxide to adjust the pH value of the water to about 10. Weigh phthalic hydrazide (1mmol) into the system, then add sodium bromide (0.5mmol), use graphite sheet as anode, stainless steel sheet as cathode, current density 10mA / cm 2 Electrolyze at a constant current, and the reaction temperature is room temperature (30°C). After 4 hours of electrification, add hydrochloric acid to adjust the pH of the system to about 1, and then filter with a Buchner funnel. Finally, heat and suspend to dry off most of the water, the heating temperature is about 80°C, and put it into the refrigerator for recrystallization. Yield: 35.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com