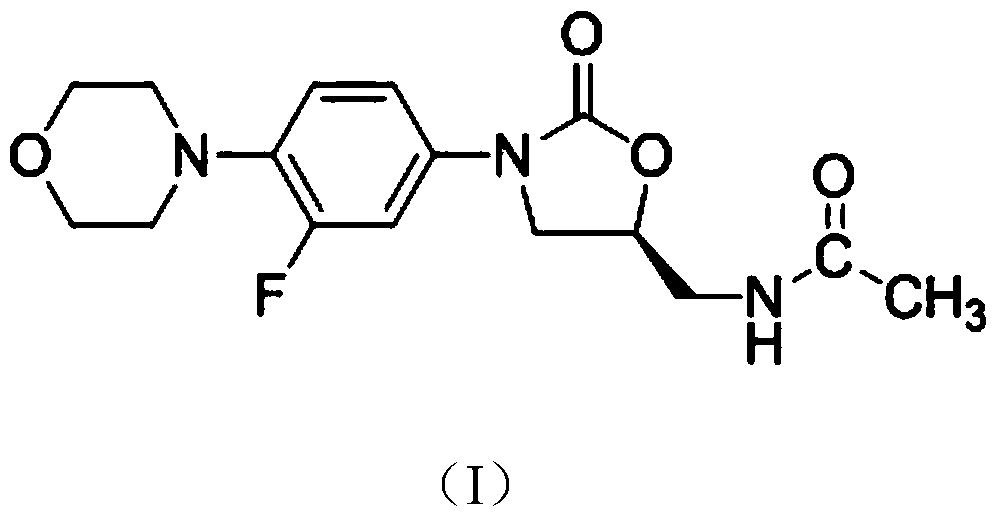
Linezolid crystal form B and preparation method and application thereof
A technology of linezolid and linezolid hydrochloride, applied in the field of medicine, can solve the problems of complicated preparation process of linezolid crystal form, affecting the personal safety of operators, difficult mixing of active substances and auxiliary materials, etc. Solubility and dissolution rate, uniform distribution, good drug dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Preparation of Linezolid Form B
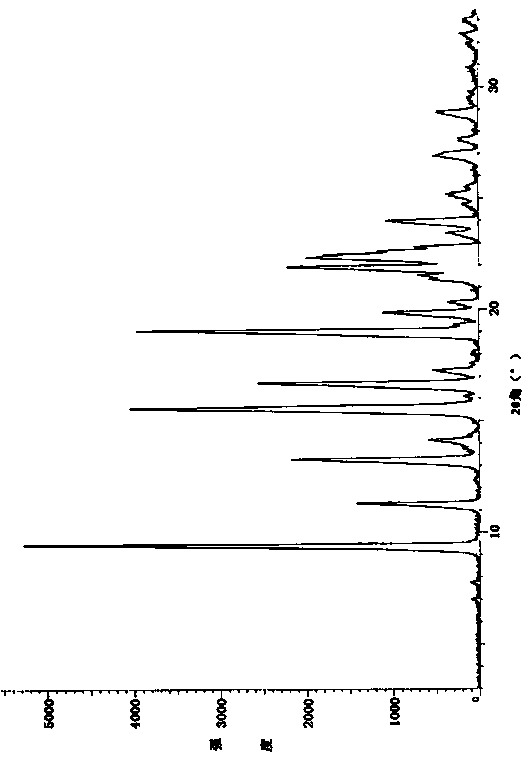
[0046] Put 2.5g of linezolid hydrochloride into a reaction flask, add 100ml of water, heat to 50°C under stirring, dissolve the solid, add dropwise 10% potassium carbonate solution, and adjust the pH of the reaction solution to 9-10. The reaction solution was stirred and crystallized, cooled naturally to room temperature, and filtered. The resulting filter cake was vacuum-dried at 40-50°C for 4-6 hours to obtain Linezolid Form B, and its XRD pattern was basically as follows: figure 1 shown.
Embodiment 2
[0047] Example 2 Preparation of Linezolid Form B
[0048] 2.2 g of linezolid hydrochloride was placed in a reaction flask, 100 ml of water was added, and the reaction liquid was heated to 40° C. while stirring, and the solid dissolved. 10% potassium carbonate solution was added dropwise to adjust the pH of the reaction solution to 8-9. The reaction solution was stirred and crystallized, cooled naturally to room temperature, and filtered. The resulting filter cake was vacuum-dried at 40-50°C for 4-6 hours to obtain Linezolid Form B, and its XRD pattern was basically as follows: figure 1 shown.
Embodiment 3
[0049] Example 3 Crystal Form Stability Investigation
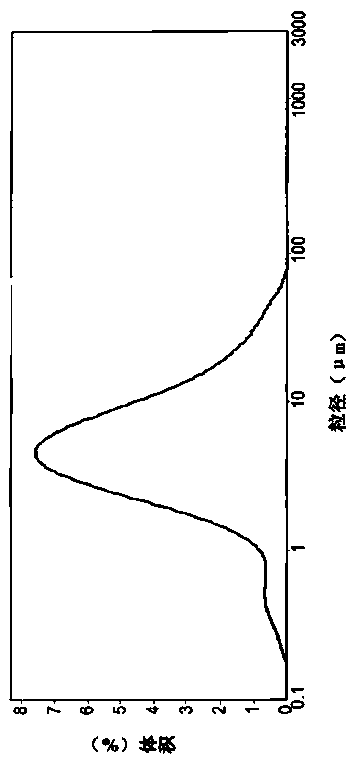
[0050] Place the linezolid crystal form B sample of the present invention in an environment with a temperature of 25°C and a relative humidity of 60%, and regularly observe the appearance changes of the crystals and detect the content of linezolid. The experimental results are shown in Table 2 :
[0051] Table 2
[0052]
[0053] The experimental results show that the crystal form B of linezolid of the present invention is stable and controllable, convenient for storage and transportation, and meets the requirements for medicinal use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com