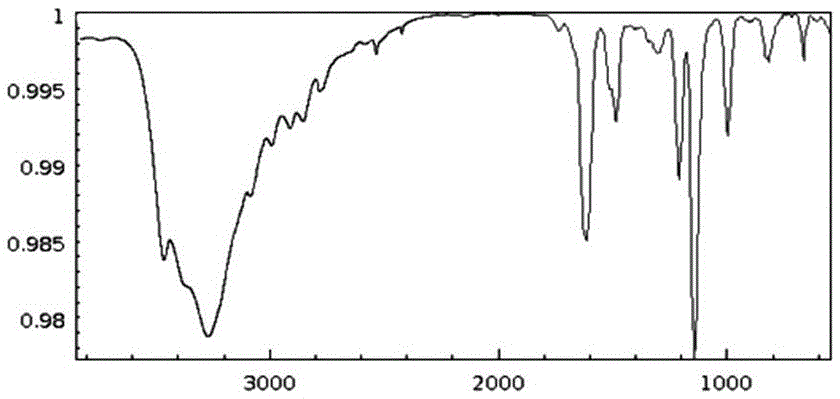
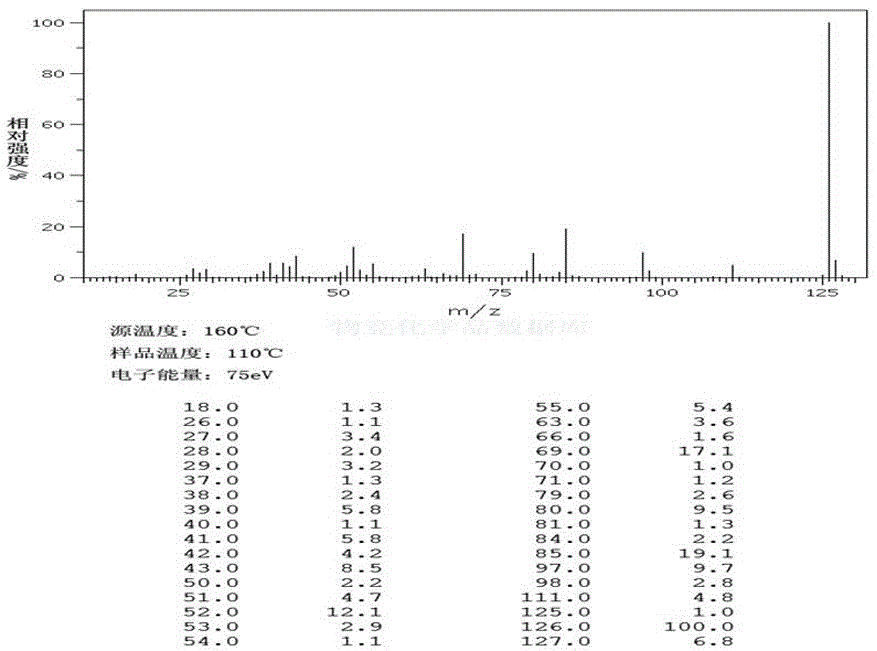
Catalytic preparation method for phloroglucinol
A technology of phloroglucinol catalysis and acid catalyst, which is applied to the preparation of peroxygen compounds, chemical instruments and methods, and the preparation of organic compounds. safe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] primary oxidation. Add 400g of TIP (96%, purity) and 400g of 2.5wt% NaOH aqueous solution, 32g of 4? molecular sieve, 10g of azobisisobutyronitrile, 7g of manganese dioxide, 2g of vanadium pentoxide and 1g of yttrium nitrate in a 2L autoclave. The stirring speed is 1250r / min, and the temperature is raised to 95°C. Add 200ml of 3.5wt% NaOH solution in the alkali dropping tank to keep the pH value of the reaction system at 9. Samples were taken every 4 hours, and after 20 hours of reaction, the material was discharged, cooled, and left to stand to obtain 748g of the aqueous phase and 482g of the organic phase (primary oxide).
[0029] Second stage oxidation. Remove the water from the primary oxide, weigh 30g and dissolve it in toluene to form a transparent solution, add it to a 500ml four-neck flask equipped with a stirrer, thermometer, and condenser, and heat up while stirring (stirring speed 1250r / min). When the temperature rises to 58°C, add H 2 SO 4 、H 2 o 2 A ...
Embodiment 2
[0033] primary oxidation. Add 400g of TIP (96%, purity) and 400g of 2wt% NaOH aqueous solution, 32g of 4? molecular sieve, 18g of cumene hydroperoxide and 2g of yttrium nitrate into a 2L autoclave, stirring at a speed of 1250r / min, and raising the temperature to 100°C. Add 200 g of 3.5wt% NaOH solution in the alkali dropping tank to keep the pH value of the reaction system at 9-10. Samples were taken every 4 hours. After 20 hours of reaction, the material was discharged, cooled, and left to stand to obtain 734 g of the aqueous phase and 489 g of the organic phase (primary oxide).
[0034] Second stage oxidation. Remove the water from the primary oxide, weigh 34g and dissolve it in toluene to form a transparent solution, add it to a 500ml four-neck flask equipped with a stirrer, thermometer, and condenser tube, and heat up under stirring (stirring speed 1200r / min). When the temperature rises to 58°C, add H 2 SO 4 、H 2 o 2 A homogeneous solution made of acid catalyst compo...
Embodiment 3
[0038] primary oxidation. In a 2L autoclave, add 400g TIP (96%, purity) and 400g 2wt% NaOH aqueous solution, 35g 4? min, the temperature was raised to 100°C. Add 200 g of 3.5wt% NaOH solution in the alkali dropping tank to keep the pH value of the reaction system at 9-10. Samples were taken every 4 hours. After 20 hours of reaction, the material was discharged, cooled, and left to stand to obtain 730 g of the aqueous phase and 492 g of the organic phase (primary oxide).
[0039] Second stage oxidation. Remove the water from the primary oxide, weigh 37g and dissolve it in toluene to form a transparent solution, add it to a 500ml four-neck flask equipped with a stirrer, a thermometer, and a condenser tube, and heat up while stirring (stirring speed 1250r / min). When the temperature rises to 58°C, add H 2 SO 4 、H 2 o 2 A homogeneous solution made of acid catalyst composed of water, ammonium persulfate and copper phthalocyanine, continue to react at 60°C for 1 hour, then sto...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap