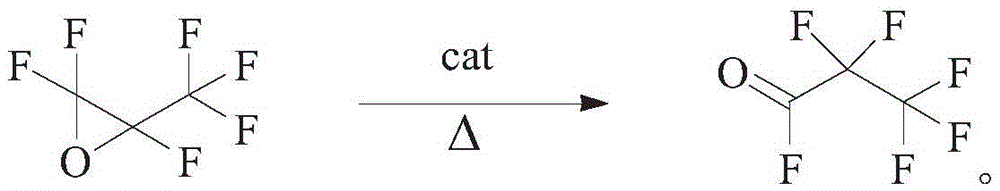
Synthetic method of perfluoropropanoyl fluoride and preparation method of catalyst applied to reaction
A technology of perfluoropropionyl fluoride and a synthesis method, which is applied in the field of fluorine-containing fine chemicals, can solve the problems of harsh reaction conditions, unfavorable industrialized production, complicated reactor design, etc., and achieves short reaction time, simple production steps and high efficiency. Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Preparation of supported catalyst:
[0030] (1) Weighing 0.05 moles of cesium fluoride (CsF) to be configured into 1 L of aqueous solution with a concentration of 0.05 mol / L, adding 50 g of activated carbon to the above solution, fully stirring, the stirring time is 5 hours;
[0031] (2) Stop stirring, put the mixed solution in a muffle furnace, evaporate the water therein, the evaporation temperature is 150 degrees Celsius, and fully dry to constant weight under vacuum and room temperature conditions to obtain a black powdery catalyst;
[0032] (3) Weighing and calculating the loading of cesium fluoride (CsF) in the supported catalyst is 12%.
[0033] 2. Synthesis of perfluoropropionyl fluoride:
[0034] (1) First, activate the supported catalyst prepared above at high temperature, set the activation temperature to 400° C., and set the activation time to 12 hours. After the activation, the system is naturally cooled down to room temperature.
[0035] (2) Adding 10...
Embodiment 2
[0040] 1. Preparation of supported catalyst:
[0041] (1) Take 0.2 moles of potassium fluoride (KF) and configure it into 500 mL of an aqueous solution with a concentration of 0.4 mol / L, add 50 g of activated carbon to the above solution, stir fully, and the stirring time is 12 hours;
[0042] (2) Stop stirring, put the mixed solution in a muffle furnace, evaporate the water therein, the evaporation temperature is 150 degrees Celsius, and fully dry to constant weight under vacuum and room temperature conditions to obtain a black powdery catalyst;
[0043] (3) Weigh and calculate the loading capacity of potassium fluoride (KF) in the supported catalyst to be 16%.
[0044] 2. Synthesis of perfluoropropionyl fluoride:
[0045] (1) Activate the supported catalyst prepared above at high temperature, set the activation temperature to 200° C., and set the activation time to 5 hours. After the activation, the system is naturally cooled down to room temperature.
[0046] (2) Adding 10g...
Embodiment 3
[0051] In order to further confirm that high-purity perfluoropropionyl fluoride can be prepared by the synthetic method of the present invention, the obtained material can be used in subsequent reactions without further purification. This example is based on Example 2 and calculated according to the conventional reaction. Weigh the produced perfluoropropionyl fluoride product, carry out esterification reaction with methanol, the reaction temperature is 50 degrees Celsius, and the reaction time is 2 hours. The product is washed with water, left to stand for liquid separation, and distilled, and the collected fractions are weighed. And calculate the yield, the yield of the prepared ester is 89%, and the purity of the prepared ester through gas chromatography analysis is 98.8%.
[0052] Content (%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com