Fluorenyl group and carboxylate combination compound and application thereof
A compound and carboxylate technology, applied in the field of catalysts, can solve the problems of harsh reaction conditions, limited application and promotion, expensive reagents, etc., and achieve the effects of mild preparation process conditions, high catalytic activity, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
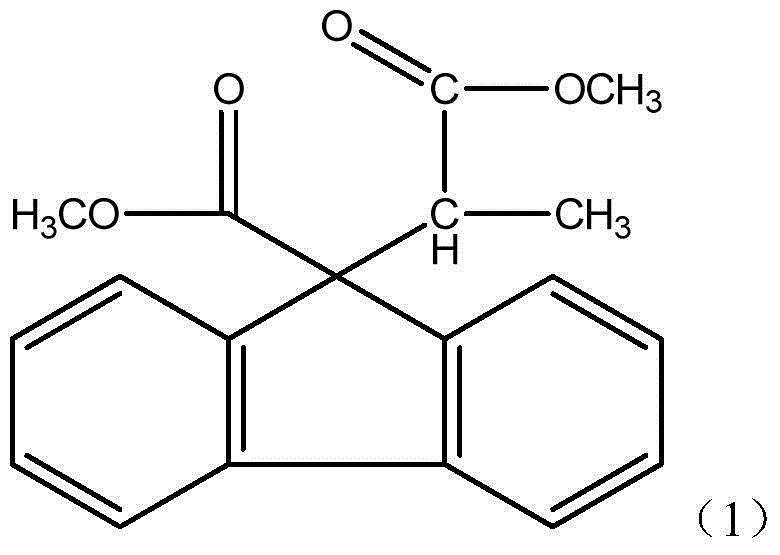
[0023] Embodiment 1: the preparation of the compound (1) that fluorene group and carboxylate are combined
[0024] The structural formula of the compound (1) in which the fluorene group and the carboxylate are combined is as follows:
[0025]
[0026] The first step: deprotonation reaction of 9H-fluorene-9-carboxylate methyl ester in anhydrous methanol solvent under the action of excess sodium methoxide, and react at room temperature for 1 hour; 9H-fluorene-9-carboxylate methyl Esters: sodium methoxide = 1:2; the amount of solvent used is to add 2L of anhydrous methanol solvent per mole of 9H-fluorene-9-carboxylate substrate.
[0027] Step 2: Add methyl α-bromopropionate to the above reaction solution, in molar ratio, methyl 9H-fluorene-9-carboxylate:methyl α-bromopropionate=1:2, react overnight at room temperature , the post-processing process is as follows:
[0028] The reaction solution was washed with CH 2 Cl 2 Extraction, the extract was washed with water, anhydrou...
Embodiment 2~4
[0031] Embodiment 2~4: the preparation of the compound (2)~(4) that fluorene group and carboxylate are combined
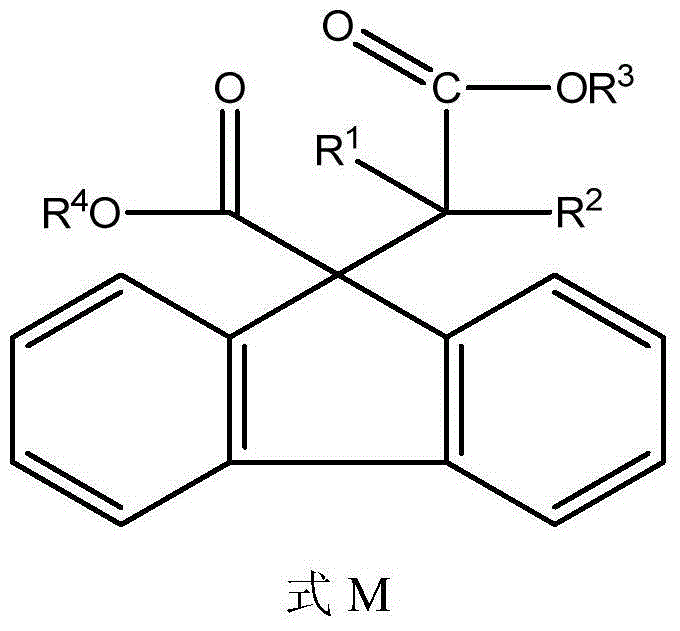
[0032] According to the method of Example 1, a series of fluorenyl groups having the general formula M are synthesized by selecting different substituted α-bromocarboxylate methyl esters and 9H-fluorene-9-carboxylate methyl esters whose structural formula is N. Compounds (2)-(4) combined with groups and methyl carboxylate. and use 1 HNMR confirmed the structure of the synthesized compound. Substituted methyl α-bromocarboxylate N and R in product formula M 1 , R 2 , R 3 , and R 4 See Table 1.
[0033]
[0034] Table 1 Combination of fluorene group and methyl carboxylate
[0035]
Embodiment 5~12
[0036] Embodiment 5~12: the preparation of the compound (5)~(12) that fluorene group and carboxylate are combined
[0037] According to the method of Example 1, different substituted α-bromocarboxylates and 9H-fluorene-9-carboxylate ethyl esters with different structural formulas Q are selected as raw materials to synthesize a series of fluorenyl groups with the general formula M Compounds (5)-(12) combined with groups and ethyl carboxylate. and use 1 HNMR confirmed the structure of the synthesized compound. Substituted ethyl α-bromocarboxylate Q and R in the product structure formula M 1 , R 2 , R 3 , and R 4 See Table 2.
[0038]
[0039] The compound of table 2 fluorene group and ethyl carboxylate combination
[0040]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Active | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com