Method for enriching and recycling bismuth from waste acid sulfide residues
A technology of sulfide slag and dirty acid, which is applied in the interdisciplinary fields of metallurgical engineering and environmental engineering, can solve the problems of waste of bismuth resources, achieve efficient recovery, reduce treatment costs and environmental risks, and reduce energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
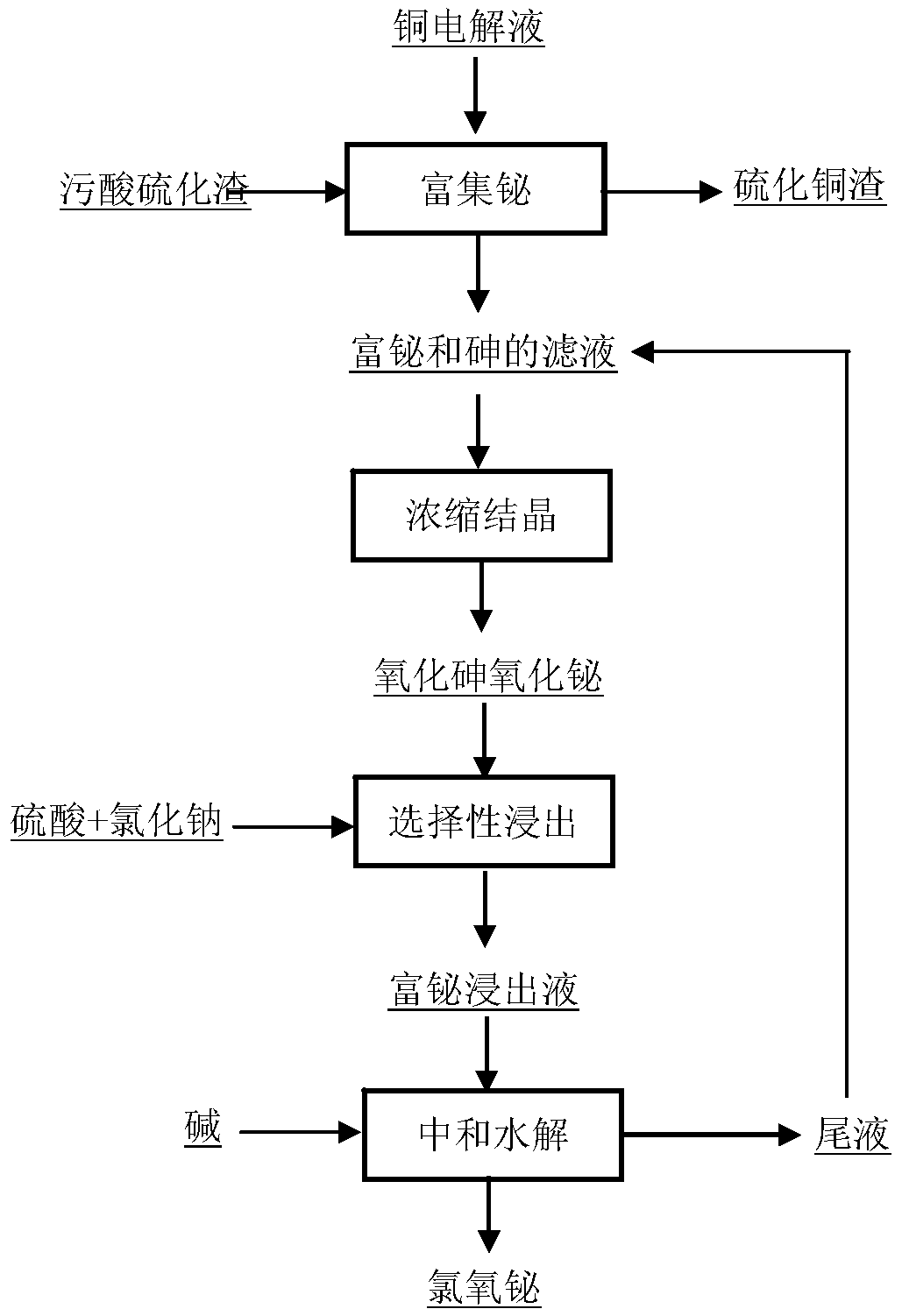
[0023] Take 500ml of electrolyte solution from a copper smelter, add polluted acid sulfide slag with bismuth content of 0.12% according to the ratio of As / Cu molar ratio 2, stir and mix well, react at 70°C for 3h, filter to obtain bismuth-rich filtrate, in the solution The concentration of bismuth increased from 0.43g / L to 1.22g / L, achieving the purpose of enriching bismuth. Evaporate and concentrate the bismuth-rich filtrate to a sulfuric acid concentration of 900g / L, cool and crystallize at 25°C for 2 hours, filter to obtain arsenic oxide and bismuth oxide mixture containing 1.8% bismuth, add sulfuric acid to the mixture at a solid-to-liquid mass ratio of 2:1 and sodium chloride solution, wherein the concentration of sulfuric acid is 5mol / L, the concentration of chloride ion is 2mol / L, leaching at 50°C for 2h, after the leaching is completed, the bismuth-rich leaching solution with a bismuth concentration of 5.6g / L is filtered, and poured into the bismuth-rich leaching soluti...
Embodiment 2
[0025] Take 500mL of copper electrolyte and add the polluted acid sulfide slag with a bismuth content of 0.14% according to the ratio of As / Cu molar ratio of 1.5, stir and mix well, react at 80°C for 2h, filter to obtain a bismuth-rich filtrate, and the concentration of bismuth in the solution is given by 0.43g / L rose to 1.75g / L, achieving the purpose of enriching bismuth. Evaporate and concentrate the bismuth-rich filtrate to a sulfuric acid concentration of 800g / L, cool and crystallize at 20°C for 3 hours, filter to obtain arsenic oxide and bismuth oxide mixture containing 2.1% bismuth, add sulfuric acid to the mixture at a solid-to-liquid mass ratio of 3:1 And sodium chloride solution, wherein the concentration of sulfuric acid is 4mol / L, the concentration of chloride ion is 3mol / L, leaching at 60°C for 1h, after the leaching is completed, the bismuth-rich leachate with a bismuth concentration of 7.8g / L is filtered, and poured into the bismuth-rich leachate Sodium hydroxide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com