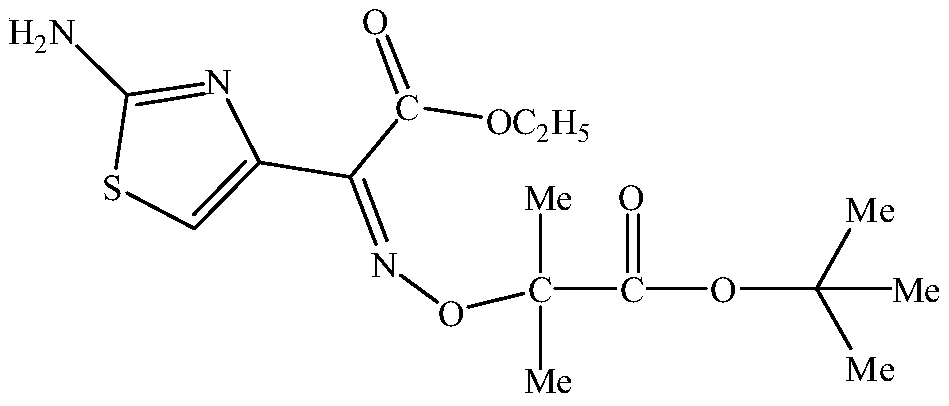
One-pot ceftazidime side-chain acid ethyl ester synthesis method
A side-chain acid ethyl ester and ceftazidime technology, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of difficult wastewater treatment, large water consumption per ton, and high environmental protection pressure, so as to achieve stable production process, reduce environmental pollution, and reduce wastewater volume effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Add 209g of ethyl 4-bromoacetoacetate and 300g of water into the reaction flask, stir and dissolve, and at -5°C, start to drop an aqueous solution of sodium nitrite (equivalent to 138g of sodium nitrite) and a mass fraction of 15% Sulfuric acid, to ensure that the pH of the solution is 1, the dripping is completed in 1 hour, heat is released during the dropping process, the temperature rises to 5°C, and the reaction is continued for 3.5 hours. After the reaction was completed, dichloromethane was added for extraction, the extract was washed with saturated potassium carbonate aqueous solution, and the organic layer was distilled under reduced pressure to obtain an oximation solution.
[0035] (2) 76g of thiourea is added to the mixed solution of 600g of water and methanol, and the oximation solution prepared in step (1) is added dropwise at 20° C., and after 3.5 hours of dripping, a solution of ethyl demethylthiaxamate is obtained; The specific gravity of the mixed l...
Embodiment 2
[0038] (1) Add 209g of ethyl 4-bromoacetoacetate and 300g of water into the reaction flask, after stirring and dissolving, at 0°C, start to dropwise add sodium nitrite (equivalent to 172g of sodium nitrite) aqueous solution and sulfuric acid with a mass fraction of 20% , to ensure that the pH of the solution is 2.5, and the dripping is completed in 1.5 hours. Heat is released during the dropping process, and the temperature rises to 10° C., and the reaction is continued for 4 hours. After the reaction was completed, dichloromethane was added for extraction, the extract was washed with saturated potassium carbonate aqueous solution, and the organic layer was distilled under reduced pressure to obtain an oximation solution.
[0039] (2) 133g of thiourea is added to the mixed solution of 1000g of water and methanol, and the oximation solution prepared in step (1) is added dropwise at 28° C., and after 4 hours, the oximation solution is obtained to obtain the ethyl desmethionine so...
Embodiment 3
[0042] (1) Add 209g of ethyl 4-bromoacetoacetate and 300g of water into the reaction flask, after stirring and dissolving, at 5°C, start to dropwise add sodium nitrite (equivalent to 207g of sodium nitrite) aqueous solution and sulfuric acid with a mass fraction of 25% , to ensure that the pH of the solution is 3.5, after 2 hours of dripping, heat is released during the dropping process, the temperature rises to 20 ° C, and the reaction is continued for 4.5 hours. After the reaction was completed, dichloromethane was added for extraction, the extract was washed with saturated potassium carbonate aqueous solution, and the organic layer was distilled under reduced pressure to obtain an oximation solution.
[0043] (2) 190g of thiourea is added to the mixed solution of 1500g of water and methanol, and the oximation solution prepared in step (1) is added dropwise at 35° C., and after 4.5 hours, the oximation solution is obtained to obtain the ethyl desmethionine solution; The spec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com