Nanosuspension of P2X7 receptor antagonist employing isoquinoline as basic skeleton and preparation method of nanosuspension
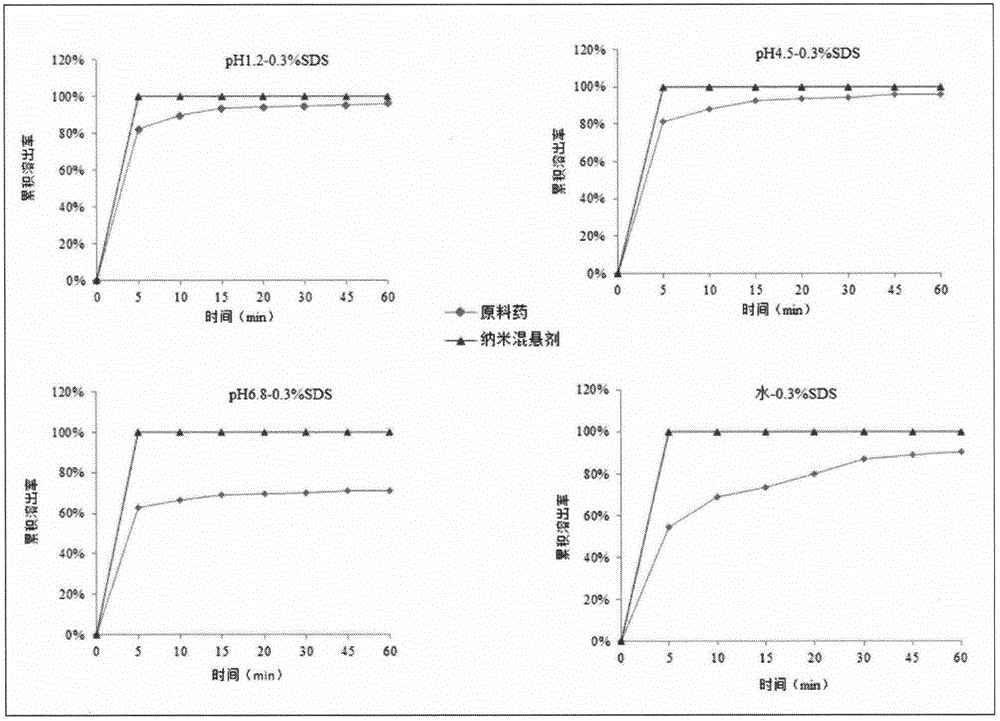
A technology of receptor antagonists and nanosuspensions, applied in the direction of anti-inflammatory agents, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of limiting the clinical application of drugs and increasing the effect of bioavailability , drug precipitation and other issues, to achieve the effect of improving the in vitro dissolution rate, good application prospects, and increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
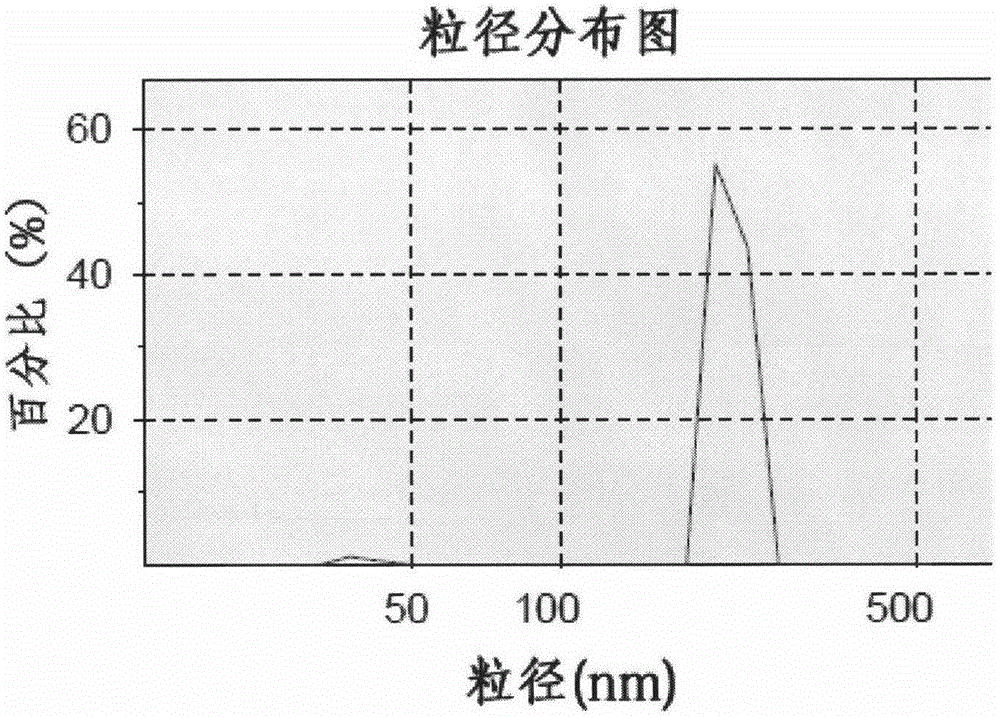
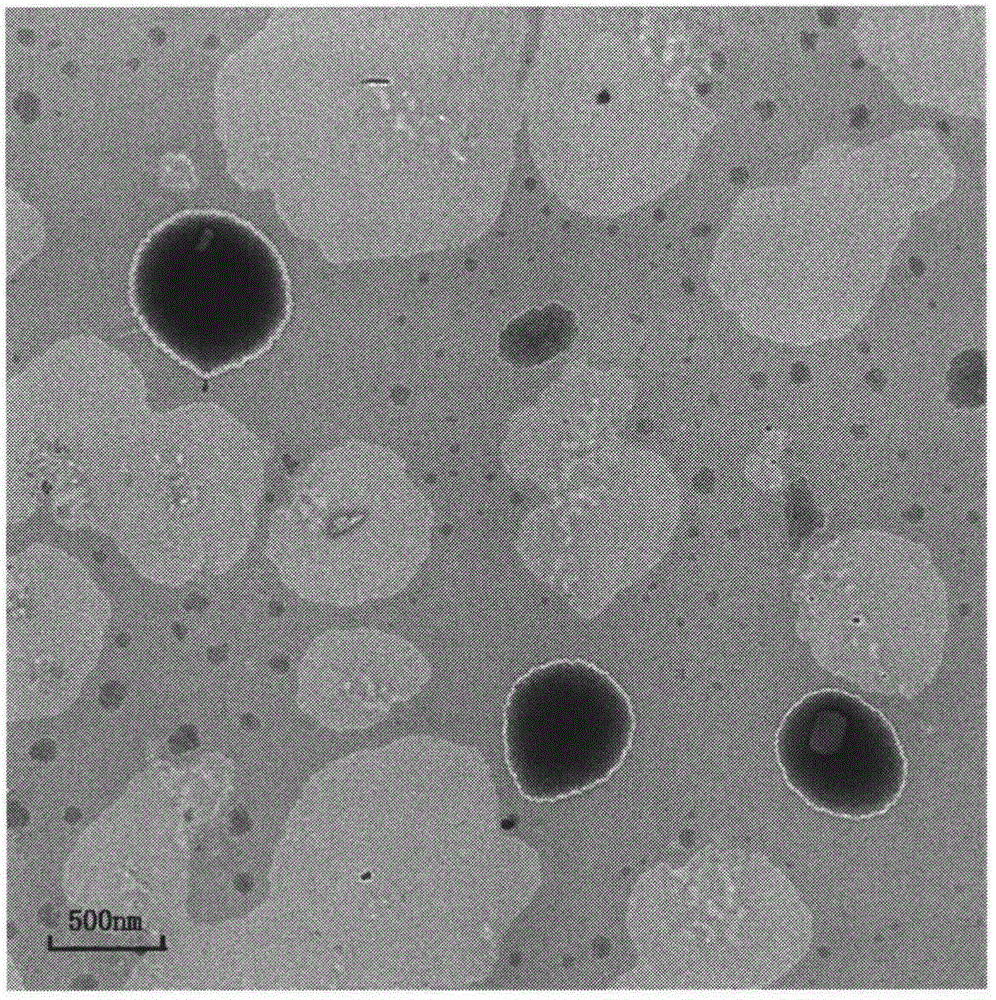
[0029] Weigh 100 mg of hydroxypropyl methyl cellulose and 10 mg of sodium lauryl sulfate and disperse in 100 mL of deionized water, make it fully dissolved by ultrasonic in a water bath, and use it as the water phase; weigh 100 mg of micronized crude drug and dissolve in 5 mL of methanol, vortex Rotate and mix well, as the organic phase. Under the condition of ice bath and high-speed stirring, slowly add the organic phase dropwise to the water phase, continue stirring for 2h, then rotate and evaporate at 40℃ to remove the organic solvent, and homogenize the resulting initial suspension at a pressure of 1200bar , Circulate for 10min, and get it. The average particle size of the nanosuspension prepared by this method is 188.4 nm, and the polydispersity coefficient PI is 0.47.
Embodiment 2
[0031] Weigh 200mg of polyvinylpyrrolidone K30 and 5mg of sodium lauryl sulfate and disperse in 100mL of deionized water, make it fully dissolved by ultrasonic in a water bath, as the water phase; Weigh 100mg of micronized crude drug in 3mL of methanol, vortex and mix, As the organic phase. Under the condition of ice bath and high-speed stirring, the organic phase was slowly added dropwise to the water phase, and then the organic solvent was removed by rotary evaporation at 40°C, and the resulting initial suspension was homogenized at a pressure of 800 bar under high pressure and circulated for 8 min. Immediately. The average particle size of the nanosuspension prepared by this method is 432.9 nm, and the polydispersity coefficient PI is 0.51.
Embodiment 3
[0033] Weigh 50 mg of sodium carboxymethyl cellulose and disperse in 100 mL of deionized water, make it fully dissolved by ultrasonic in a water bath, as the water phase; weigh 100 mg of the micronized crude drug in 5 mL of methanol, vortex and mix, as the organic phase. Under the condition of ice bath and high-speed stirring, the organic phase was slowly added dropwise to the water phase, and then the organic solvent was removed by rotary evaporation at 42°C. The resulting initial suspension was homogenized at a pressure of 1000 bar under high pressure and circulated for 5 minutes. Immediately. The average particle size of the nanosuspension prepared by this method is 559.2nm, and the polydispersity coefficient PI is 0.44.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com