Preparation method and application of alkannin analogue based on anthraquinone structure
A technology for shikonin and analogs, which is applied in the field of preparation of shikonin analogs, can solve problems such as failure to provide naphthenic ring modification, and achieve the effects of simple preparation method, reduced toxicity, and rich structure-activity relationship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
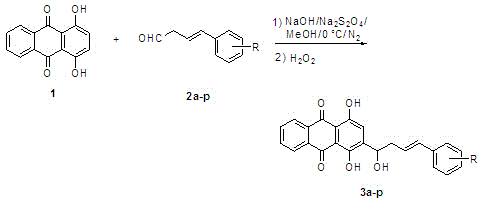
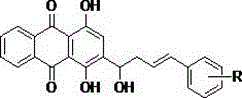
[0023] General synthesis of 2-(4-substituted phenyl-1-hydroxy-3-butene)-1,4-dihydroxy-9,10-anthraquinone derivatives 3a-p:
[0024] 1,4-Dihydroxyanthraquinone 1 (0.5mmol) was dissolved in methanol (10ml), and sodium hydroxide solution (1N, 2.5ml) was added. Under nitrogen protection, sodium dithionite (1.0 mmol) dissolved in water (2 mL) was added. After stirring for 10 minutes, β,γ-unsaturated aldehyde 2 (1.0 mmol) was added and reacted at 0°C for 3 hours. The reaction solution was poured into 10 mL of ice water added with 30% hydrogen peroxide (2 mL), and stirred for 10 minutes. Add hydrochloric acid solution (3N, 1mL) to acidify, extract with dichloromethane, wash with saturated sodium bicarbonate solution, wash with water, wash with saturated brine, and dry over anhydrous magnesium sulfate. Separation by column chromatography gave orange-red solid 3.
[0025] 2-(4-Phenyl-1-hydroxy-3-butene)-1,4-dihydroxy-9,10-anthraquinone 3a
[0026] Yield 80%; mp130-132oC; 1 HNMR (4...
Embodiment 2
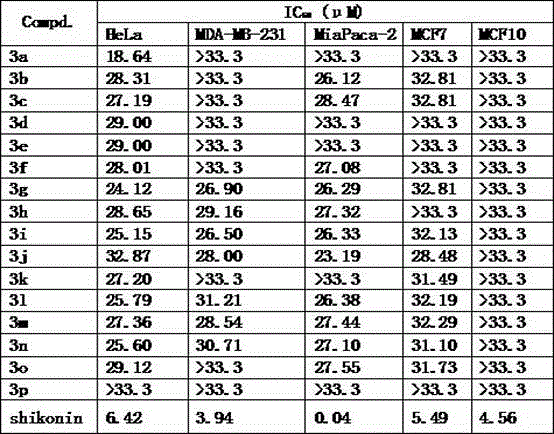
[0057] Example 2: In vitro cell activity test of compounds 3a-p.
[0058] The growth inhibitory effect of the compound of the present invention on human cervical cancer cell HeLa, human breast cancer cell MDA-MB-231, human pancreatic cancer cell MiaPaca-2, human breast cancer cell MCF-7 and human normal breast epithelial cell MCF10 was determined. According to the inhibition rate of the target compound on cell growth at different concentrations, the concentration of the compound that inhibits cell growth to 50% was calculated, and shikonin was used as a positive control. The results are listed in Table 2, with IC 50 Value representation.
[0059] Table 2 Toxicity IC of target compounds to different tumor cells and normal cells 50 (μM)
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com