Protein-polyphenol covalent compound and preparation method and application thereof
A protein and complex technology, applied in the field of food science and food additives, can solve the problems of poor physical and chemical stability, and achieve the effects of good safety, simple preparation method, high physical and chemical stability and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
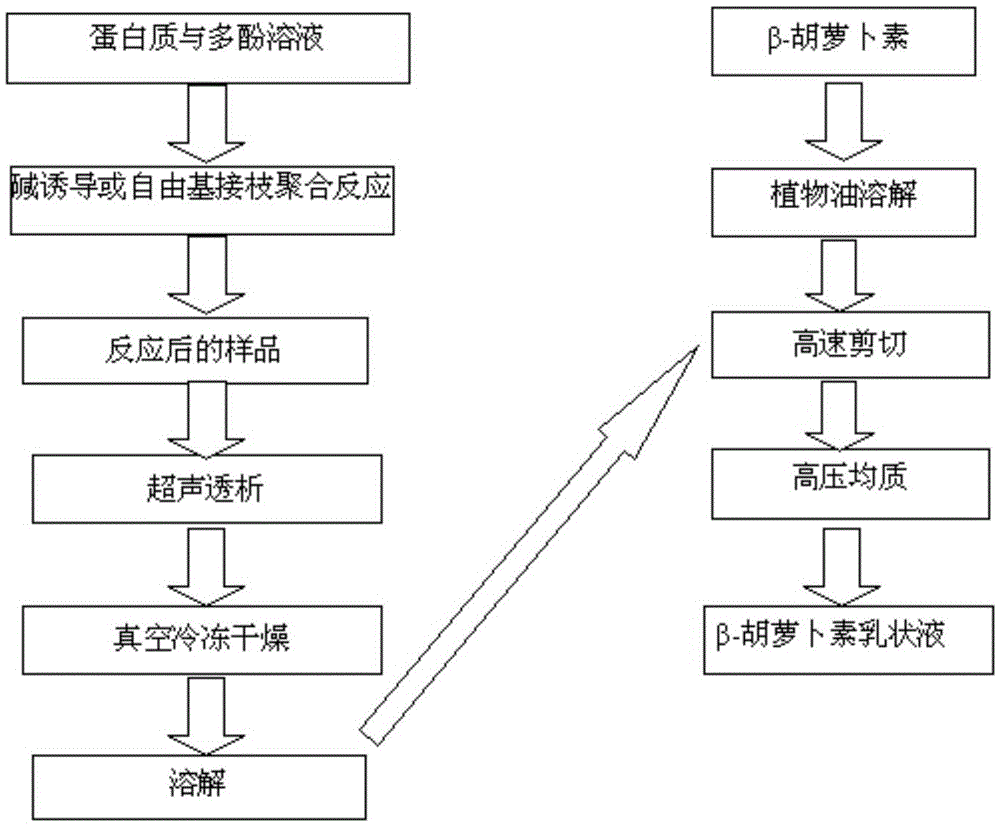
[0033] First use 0.5mol / L sodium hydroxide solution to adjust the pH value of deionized water to 9; then weigh 0.5g α-lactalbumin, β-lactoglobulin, lactoferrin and sodium caseinate, and dissolve them in 50mL pH. In deionized water with a value of 9, four milk protein solutions are formed; 0.1g of epigallocatechin gallate (EGCG) is dissolved in 50 mL of deionized water with a pH of 9 to form an EGCG solution;
[0034] Under the condition of constant stirring at 120 rpm, the EGCG solution was added to the four milk protein solutions and air was introduced to form a reaction system; then the reaction system was stirred at a constant speed of 120 rpm at room temperature for 24 hours for reaction; The sample was dialyzed at 4°C and 60KHz ultrasonic frequency for 48h, and the water was changed every 3h to remove unreacted free EGCG, and then the remaining liquid in the dialysis bag was freeze-dried to obtain four milk protein-EGCG covalent complexes Things.
[0035] Differential scannin...
Embodiment 2
[0040] Take the four milk proteins and the four milk protein-EGCG covalent complexes in Example 1, and use 10mM sodium phosphate buffer (pH=7.0) to prepare 0.02-0.3mg / mL protein solution or protein-poly Phenol covalent complex solution, while preparing 8mM 8-anilino-1-naphthalenesulfonic acid (ANS) solution. Add 20 μL of ANS solution to 4 mL of the above protein solution and mix well. Excite the fluorescence spectrum at a wavelength of 390nm, measure the fluorescence intensity at a wavelength of 470nm, and plot the relative fluorescence intensity of the sample against the protein concentration. The linear slope of the initial segment is the surface hydrophobicity index of the sample. The measurement results are shown in Table 2. Show.
[0041] Table 2: Comparison of the surface hydrophobicity index of milk protein and milk protein-EGCG covalent complex
[0042]
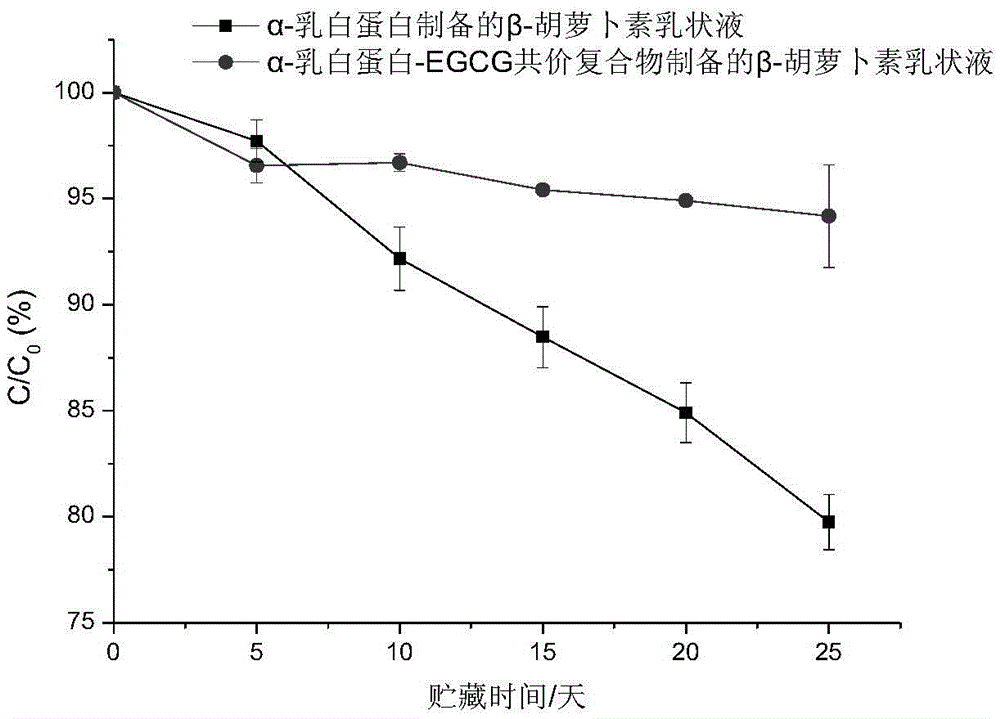
[0043] It can be seen from Table 2 that the surface hydrophobicity index of milk protein is significantly reduced afte...
Embodiment 3
[0045] Take the four milk proteins and the four milk protein-EGCG covalent complexes in Example 1, and use 10mM sodium phosphate buffer (pH=7.0) to prepare 0.02-0.3mg / mL protein solutions; The solution was added to 3 mL of ABTS solution, and after reacting for 60 minutes at room temperature in the dark, the absorbance was measured at a wavelength of 734 nm. The ability of the sample to scavenge ABTS free radicals is compared with that of water-soluble vitamin E (Trolox). The ability of the sample to scavenge ABTS free radicals is expressed by Trolox equivalent (nmolTE / g dry basis). The measurement results are shown in Table 3.
[0046] Table 3: Comparison of ABTS free radical scavenging ability of milk protein and milk protein-EGCG covalent complex
[0047]
[0048] Note: The unit of ABTS free radical scavenging ability in the above table is nmolTE / g dry basis.
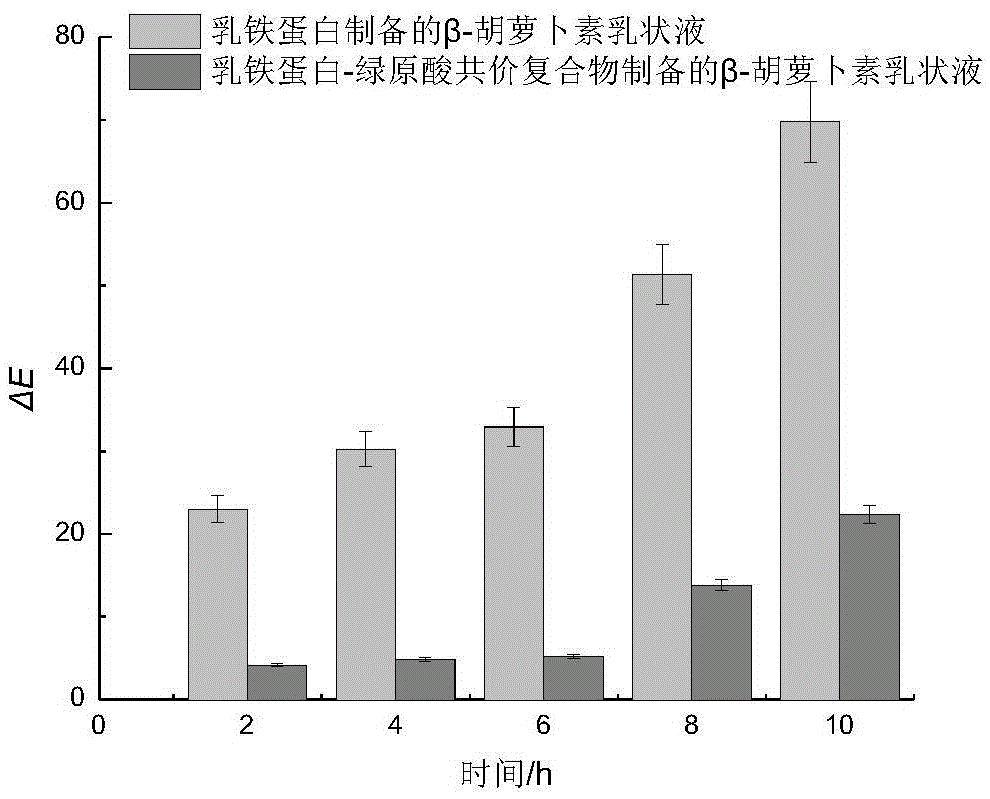
[0049] It can be seen from Table 3 that the ABTS free radical scavenging ability of the milk protein-EGCG covalent comple...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com