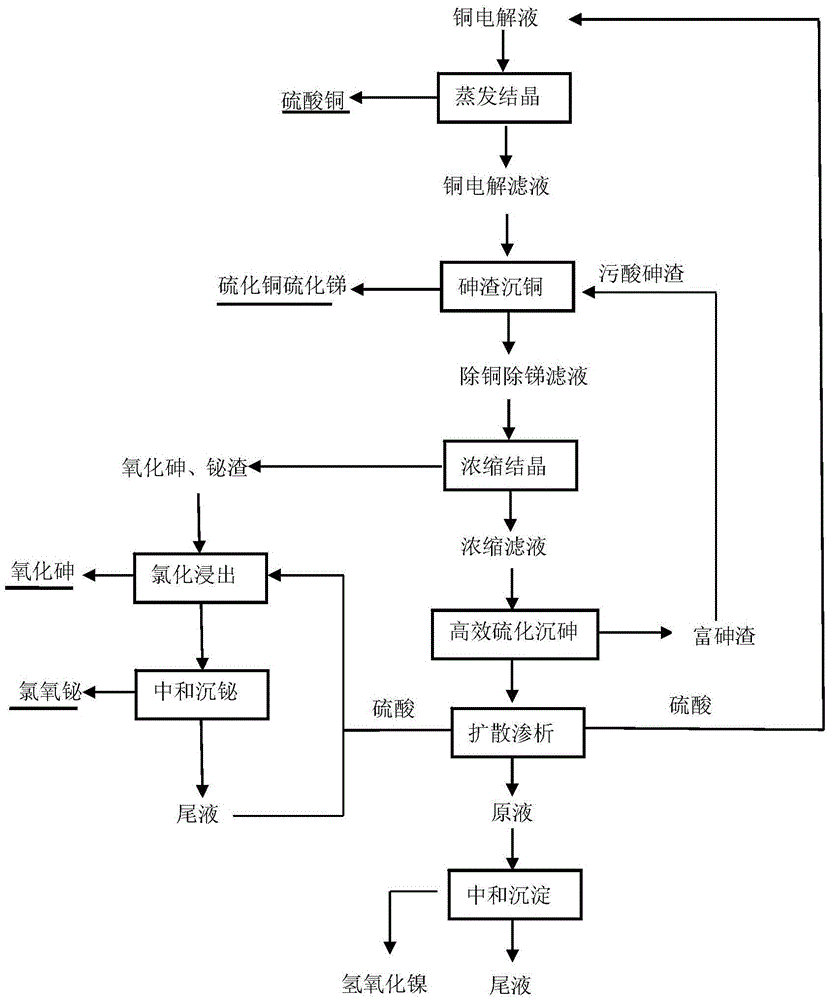
Method for purifying and recovering valuable metal by copper electrolyte
A copper electrolyte and valuable metal technology, applied in the intersection of metallurgical engineering and environmental engineering, can solve the problems of high cost, high energy consumption, difficult recovery of valuable metals, etc., and achieve efficient recovery, low energy consumption, and reduced production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
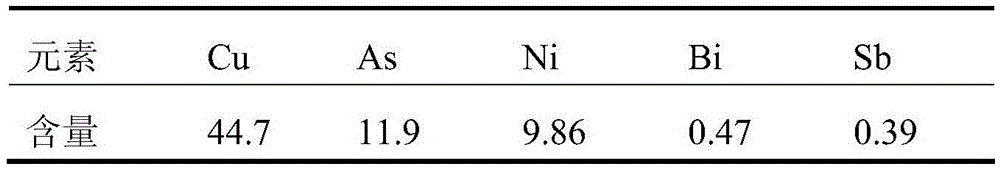
[0030] Get 500ml of electrolyte in a copper smelter, the concentration of sulfuric acid is 289g / L, and other main elements and their contents are as follows:
[0031] Table 1 Main elements and concentration of copper electrolyte (g / L)
[0032]
[0033] After evaporating and concentrating the above electrolytic solution to 250ml, cool and crystallize and filter to obtain 29g copper sulfate pentahydrate. The copper concentration in the filtrate is about 60g / L, and the concentration of other main elements is doubled. Cu) was added to the filtrate at a ratio of 1.2, reacted at 80°C for 3 hours, and filtered to obtain the precipitation of copper sulfide and antimony sulfide and the filtrate for removing copper and antimony. The concentration was 97.8mg / L, the total recoveries of copper and antimony were 99.9% and 87.5%, respectively. Evaporate and concentrate the above-mentioned copper and antimony removal filtrate to a sulfuric acid concentration of 800g / L, then cool and stand...
Embodiment 2
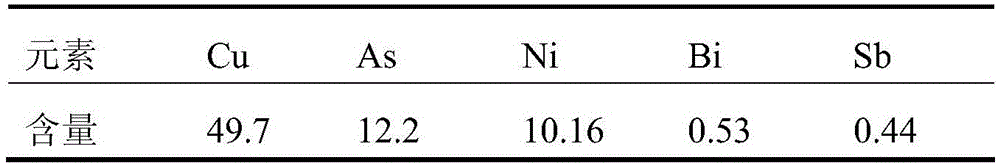
[0035] Get 500ml of electrolyte in a copper smelter, the concentration of sulfuric acid is 267g / L, and other main elements and their contents are as follows:
[0036] Table 1 Main elements and concentration of copper electrolyte (g / L)
[0037]
[0038] After evaporating and concentrating the above electrolytic solution to 250ml, cool and crystallize and filter to obtain 30g copper sulfate pentahydrate. The copper concentration in the filtrate is about 64g / L, and the concentration of other main elements is doubled. Cu) was added to the filtrate at a ratio of 1.3, reacted at 80°C for 3 hours, and filtered to obtain the precipitation of copper sulfide and antimony sulfide and the filtrate for removing copper and antimony. The concentration was 90.8mg / L, the total recoveries of copper and antimony were 99.9% and 89.7%, respectively. Evaporate and concentrate the above-mentioned copper and antimony removal filtrate to a sulfuric acid concentration of 850g / L, then cool and stand...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com