Preparation method of novel triazole antifungal drug
A compound, posaconazole technology, applied in the field of preparation of novel triazole antifungal drugs, can solve problems such as difficulty in ensuring product quality standards, increased difficulty in process control, high requirements for reaction equipment, etc. The effect of increased impurities, low equipment requirements and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
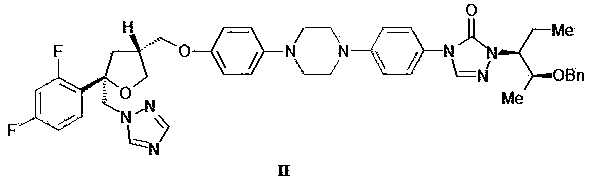
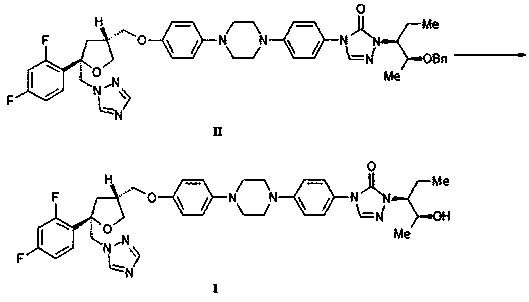
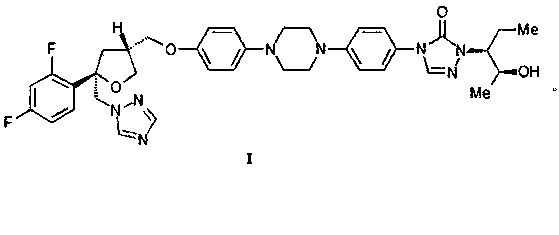
[0037] Example 1 Preparation of posaconazole (compound I)
[0038]
[0039] Add concentrated hydrochloric acid (40ml) into a 100ml three-neck flask, then add compound II (8.0g, 10.1mmol), stir to dissolve and react at 60~65°C for 2~4h. After the reaction, cool to room temperature, add water (40ml) to dilute, and wash twice with dichloromethane (50ml×2) to remove impurities. Dichloromethane (50ml) was added to the remaining aqueous phase, and then 20% sodium hydroxide solution was added to adjust the pH to 6~7, and the organic phase was collected by liquid separation. The organic phase was washed with 20% sodium chloride solution and concentrated. Dissolve the concentrate in ethanol (50ml), add medicinal activated carbon (0.15g), reflux and stir for 2~3h, then filter while it is hot, add water (7ml) to the filtrate, reflux and stir, cool and crystallize, then filter. The filter cake was crystallized with ethanol / water=9:1 (70ml). After filtration, the filter cake was vacu...
Embodiment 2
[0040] Example 2 Preparation of posaconazole (compound I)
[0041]Add concentrated hydrochloric acid (40ml) into a 100ml three-neck flask, then add compound II (8.0g, 10.1mmol), stir to dissolve and react at 60~65°C for 2~4h. After the reaction, cool to room temperature, add water to dilute to pH about 3, and wash twice with toluene (50ml×2) to remove impurities. Add toluene (50ml) to the remaining aqueous phase, then add 5% potassium carbonate solution to adjust the pH to about 6, and collect the organic phase by liquid separation. The organic phase is washed with sodium chloride solution and concentrated. Dissolve the concentrate in ethanol (56ml), add medicinal activated carbon (0.15g), reflux and stir for 2-3 hours, then filter while it is hot, add water (9ml) to the filtrate, reflux and stir, cool and crystallize and then filter. The filter cake was crystallized with ethanol / water=6:1 (7ml / g). After filtration, the filter cake was vacuum-dried (55~60°C) to obtain 5.78...
Embodiment 3
[0042] Example 3 Preparation of posaconazole (compound I)
[0043] Add concentrated hydrochloric acid (40ml) into a 100ml three-neck flask, then add compound II (8.0g, 10.1mmol), stir to dissolve and react at 60~65°C for 2~4h. After the reaction, cool to room temperature, add water (120ml) to dilute, and wash twice with ethyl acetate (50ml×2) to remove impurities. Ethyl acetate (50ml) was added to the remaining aqueous phase, and then 20% ammonia water was added to adjust the pH to 5, and the organic phase was collected by liquid separation. The organic phase is washed with sodium chloride solution and concentrated. Dissolve the concentrate in ethanol (240ml), add medicinal activated carbon (0.15g), reflux and stir for 2~3h, then filter while it is hot, add water (16ml) to the filtrate, reflux and stir, cool and crystallize and then filter. The filter cake was crystallized with ethanol / water=15:1 (116ml) (15ml / g). After filtration, the filter cake was vacuum-dried (55~60°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com