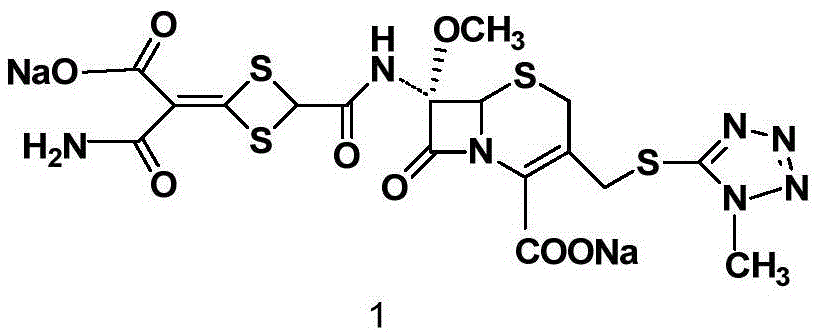
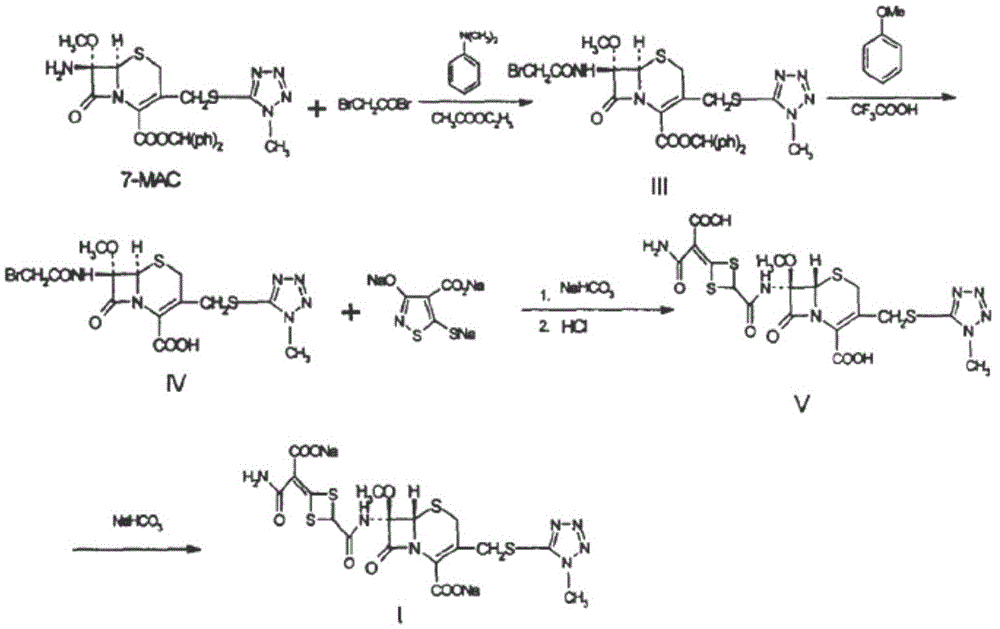
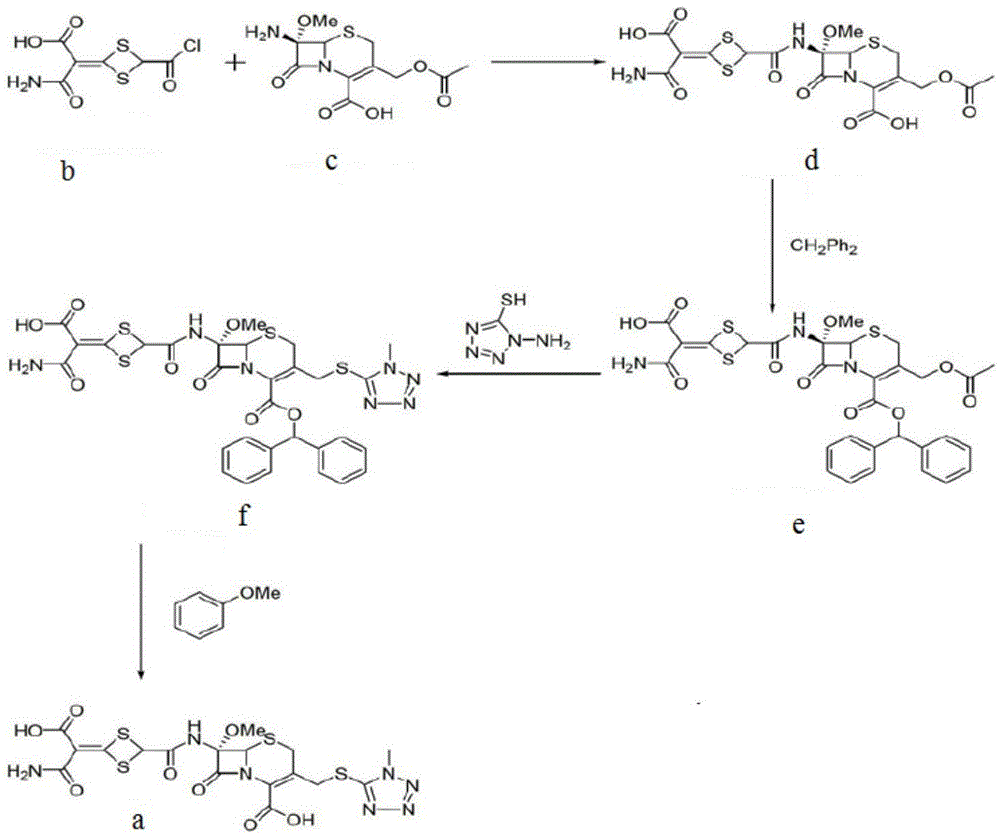
Cefotetan disodium and preparation method of intermediate of cefotetan disodium
A technology of cefotetan disodium and dichloromethane, applied in directions such as organic chemistry, can solve problems such as unfavorable industrialized production, and achieve the effects of low cost, high yield and simple process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of 4-(1-amino-3-tert-butoxy-1,3-dioxo-2-enyl)-1,3-dithietane-2-carboxylic acid (2)
[0033] At room temperature, add 4-carboxy-5-mercapto-3-hydroxy-isothiazole trisodium (5) (24.31g, 0.10mol) into 300ml of dichloromethane, stir to dissolve, add 6mol / L (about 65ml) hydrochloric acid Stir to make the pH to 2-3, add 297mg DMAP and continue to stir for 20 minutes, then add 50ml tert-butanol, 6ml pyridine, Boc 2 O (21.83g, 0.10mol), stirred and reacted at room temperature in a dry environment for 18h, HPLC monitored that the reaction was complete, and the solvent was removed by rotary evaporation to obtain an oily substance that was extracted with ethyl acetate, and the organic layer was dried with anhydrous magnesium sulfate and concentrated. , recrystallized from ethanol to obtain intermediate 6 (24.11 g), with a molar yield of 87% and a HPLC purity of 99.2%.
[0034] Take compound 6 (23.89g, 0.086mol), add 250ml of acetone, stir until dissolved, cool to 5-10°...
Embodiment 2
[0036] Preparation of Compound 4
[0037] Add compound 2 (14.56g, 0.05mol), compound 3 (31.48g, 0.06mol) and dichloromethane 1000ml in turn in the reaction flask, stir to make it dissolve; add 1-hydroxybenzotriazole (HOBT) (8.11g , 0.06mol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC) (9.31g, 0.06mol), the mixture was stirred at room temperature for a reaction time of 18h, and the HPLC monitoring reaction was complete, and then The dichloromethane was removed by rotary evaporation, and 1500ml of ethyl acetate and 1200ml of saturated sodium bicarbonate solution were added to the resulting oil, and the layers were separated. The organic layer was dried over anhydrous sodium sulfate and filtered. After the filtrate was concentrated, it was recrystallized with ether to obtain a white solid compound 4 (39.10 g), molar yield 98%, HPLC purity 99.6%.
Embodiment 3
[0039] Preparation of Compound 4
[0040] Add compound 2 (14.56g, 0.05mol), compound 3 (26.23g, 0.05mol) and dichloromethane 1000ml in turn in reaction bottle, stir to make it dissolve; Add N,N-diisopropylethylamine (7.75g , 0.06mol) and dichlorinated phenyl phosphate (PDCP) (12.66g, 0.06mol), the mixture was stirred at room temperature for a reaction time of 32h, and the HPLC monitoring reaction was complete, then rotary evaporation removed dichloromethane, and the resulting oil was added to 1500ml ethyl acetate Esters and 1200ml saturated sodium bicarbonate solution were separated. The organic layer was dried over anhydrous sodium sulfate and filtered. After the filtrate was concentrated, it was recrystallized with ether to obtain white solid compound 4 (37.11g). The molar yield was 93%, and the HPLC purity was 99.5g. %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com