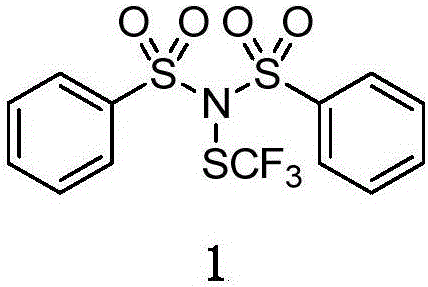
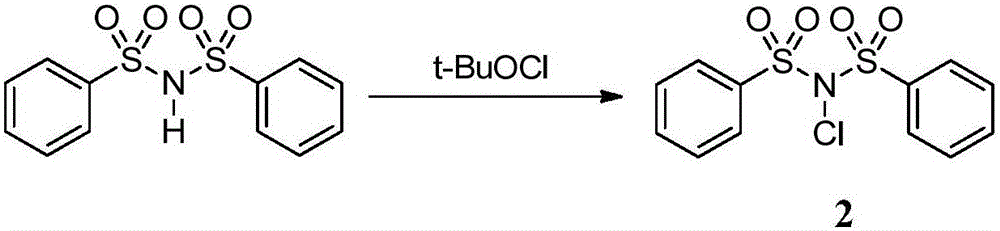
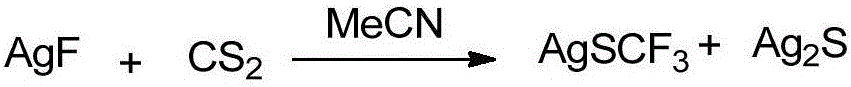Trifluoromethylthiolation reagent and its preparation method and use in asymmetric trifluoromethylthiolation reaction
A technology of trifluoromethylthio and reagents, which is applied in the preparation of sulfonic acid amide, organic chemical methods, organic chemistry, etc., can solve the problem of dose reduction, and achieve the effects of simple operation, high activity, and convenient storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) In the glove box, put 7.5g of silver fluoride into the oven-dried reaction bottle, cap the bottle stopper and take it out of the glove box. Add 40 mL redistilled acetonitrile and 12 mL carbon disulfide in a nitrogen atmosphere, quickly install a reflux condenser and reflux overnight at 80°C. Then carbon disulfide was distilled off, and the remaining acetonitrile was spin-dried with a rotary evaporator to obtain a black solid. The black solid was then dissolved and washed with ethyl acetate and filtered through celite with multiple washes. The filtrate was spin-dried under dark conditions to obtain a viscous solid. After dissolving the viscous solid with 30 drops of acetonitrile, slowly add 90 mL of ether into the bottle along the bottle wall. After the mixture was allowed to stand at room temperature for 12 hours, it was placed at -20°C for 24 hours. Pour off the ether and continue to spin dry to obtain 4 g of off-white solid silver fluoride with metallic luster. ...
Embodiment 2
[0031] (1) In the glove box, 15g of silver fluoride is packed into the oven-dried reaction bottle, and the bottle stopper is taken out of the glove box. Add 80mL redistilled acetonitrile and 25mL carbon disulfide in a nitrogen atmosphere, quickly install the reflux condenser and reflux overnight at 80°C. Then carbon disulfide was distilled off, and the remaining acetonitrile was spin-dried with a rotary evaporator to obtain a black solid. The black solid was then dissolved and washed with ethyl acetate and filtered through celite with multiple washes. The filtrate was spin-dried under dark conditions to obtain a viscous solid. After dissolving the viscous solid with 60 drops of acetonitrile, slowly add 180 mL of ether into the bottle along the bottle wall. After the mixture was allowed to stand at room temperature for 12 hours, it was placed at -20°C for 24 hours. Pour off the ether and continue to spin dry to obtain 8 g of off-white solid silver fluoride with metallic luste...
Embodiment 3
[0041] Embodiment 3: catalytic reaction example 1
[0042] Put 16.22mg (0.1mmol) of 4-phenyl-3-butenoic acid, 60mg of trifluoromethylthio reagent 1 and 8mg of catalyst in a reaction flask, add 4mL of dichloromethane and 4.4mL of TfOH at 0°C and stir for 12 hours , flash column chromatography after spinning to obtain the target product, 84% yield, 90% ee value.
[0043]
[0044] 1 HNMR (400MHz, CDCl 3 )δ7.51–7.31(m,5H),5.39(d,J=6.4Hz,1H),3.93(q,J=7.4Hz,1H),3.21(dd,J=18.2,8.4Hz,1H), 2.81(dd,J=18.2,7.4Hz,1H).
[0045] 13 CNMR (101MHz, CDCl 3 )δ172.89, 136.14, 134.45 (q, J=307.5Hz), 131.39, 129.67, 129.24, 128.34, 125.66, 125.62, 84.61, 45.83, 36.46.
[0046] 19 FNMR (377MHz, CDCl 3 )δ-39.63.
[0047] HR-ESI-MSm / zcalcd.forC 11 h 9 f 3 NaO 2 S[M+Na] + :285.0168,found:285.0173.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com