PVC (polyvinyl chloride) sole material suitable for high-temperature steam sterilization and production method thereof
A high-temperature steam sterilization and shoe sole material technology, applied in the field of medical materials, can solve the problems of physical and mechanical properties degradation, softening deformation, precipitation of additives, etc., and achieve excellent mechanical properties, excellent tear resistance, and high temperature thermal stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
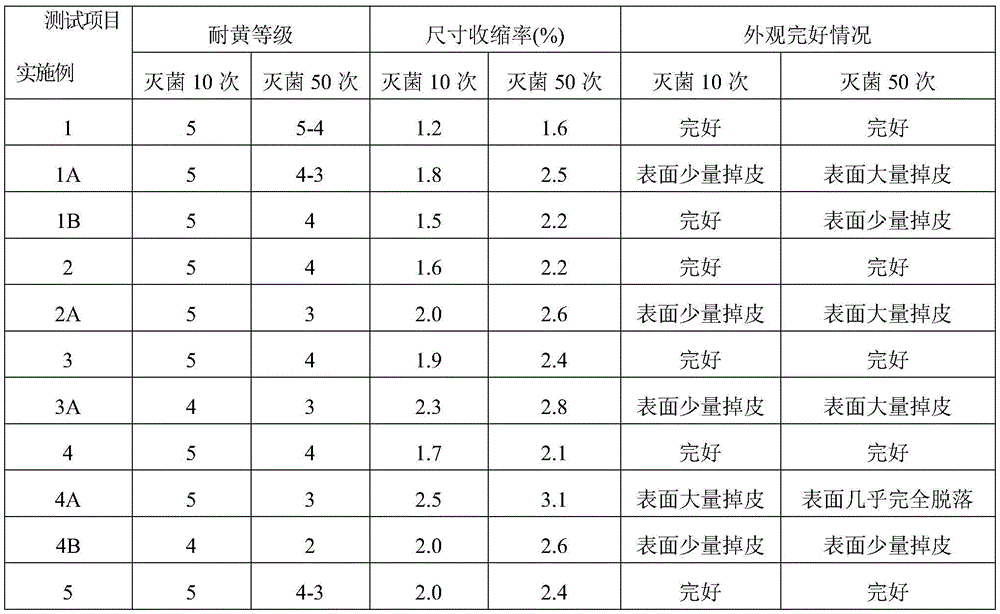
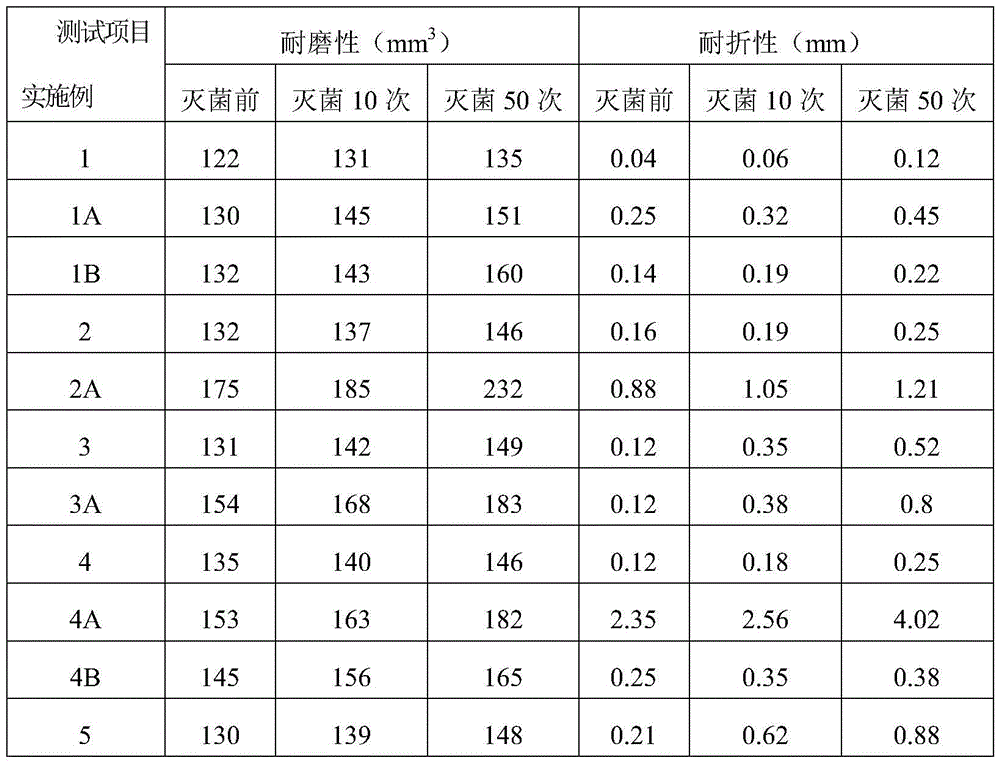
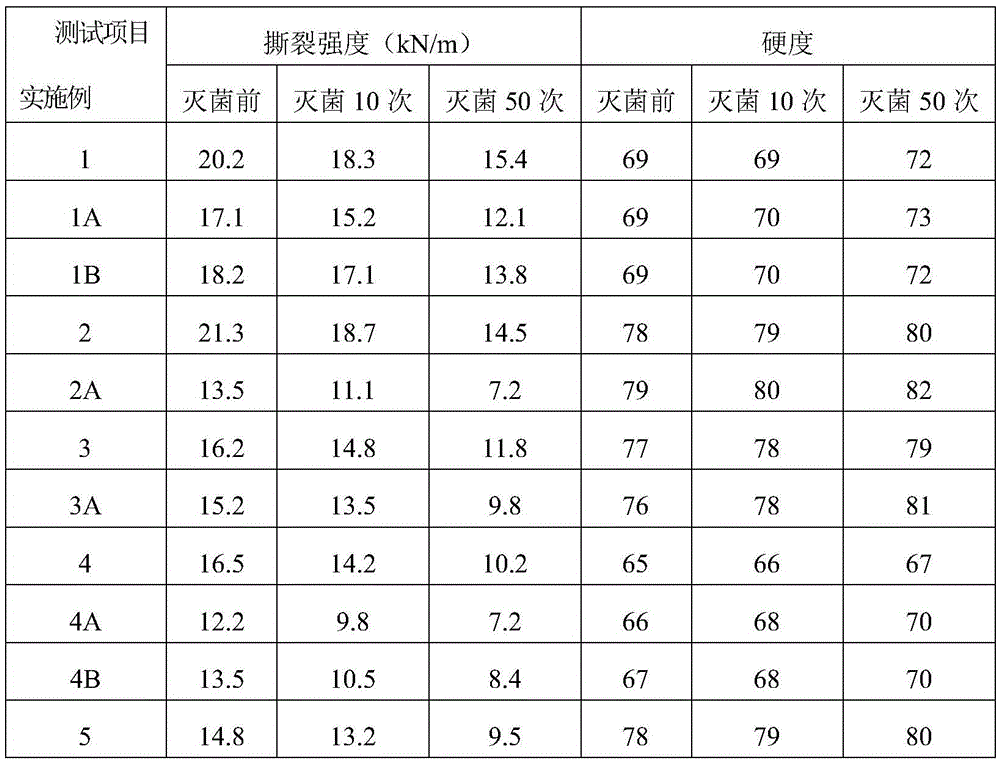
Examples
Embodiment 1
[0036] 100 parts of PVC resin, 60 parts of acetyl tributyl citrate, 10 parts of auxiliary plasticizer ElvaloyHP74110 parts, 1.2 parts of calcium stearate, 0.5 parts of zinc palmitate, 0.5 parts of dibenzoylmethane, 6 parts of epoxidized soybean oil, glycerin 2 parts of propoxy triglycidyl ether, 4.5 parts of hydroxyl-terminated hyperbranched polyamide resin, 2 parts of oxidized polyethylene wax, 4 parts of membrane split polypropylene fiber, 0.8 part of PP-g-(GMA-co-St), 2 -0.2 parts of butyl-1,2-benzisothiazolin-3-one.
[0037] The PVC resin used in this example is a PVC resin with a polymerization degree of 1250-1350 obtained by bulk polymerization, and the film-split polypropylene fibers used are 6mm in length and 600-800D in fineness. The production method of the medical PVC shoe sole material that the present embodiment can be sterilized by high temperature steam comprises the steps:
[0038] (1) Preparation of composite heat stabilizer: the mixed solution of epoxidized ...
Embodiment 2
[0049] 100 parts of PVC resin, 40 parts of triisodecyl trimellitate, 15 parts of auxiliary plasticizer ElvaloyHP44115 parts, 0.6 parts of calcium stearate, 0.2 parts of zinc stearate, 0.2 parts of stearyl benzoyl methane, epoxy 3.6 parts of soybean oil, 2.4 parts of bisphenol A diglycidyl ether, 0.4 parts of hyperbranched polysiloxane resin, 0.1 part of oxidized polyethylene wax, 2 parts of film split polyester fiber, 1.0 parts of PVC-g-MMA, inorganic nano Silver 0.1 part.
[0050] The PVC resin used in this embodiment is a PVC resin with a degree of polymerization of 1050-1150 obtained by bulk polymerization, and the length of the split polyester fiber used is 2 mm, and the fineness is 1000-1200D. The production method of the medical PVC shoe sole material that can be sterilized by high-temperature steam in this embodiment is the same as that in Example 1, and the raw materials and consumption are changed into those of the above-mentioned formula.
Embodiment 3
[0055] 100 parts of PVC resin, 50 parts of tributyl citrate, 115 parts of auxiliary plasticizer ElvaloyHP405, 1.5 parts of calcium stearate, 0.5 parts of zinc palmitate, 0.5 parts of benzoylacetone, 7 parts of epoxy soybean oil, bisphenol A 2 parts of diglycidyl ether, 2 parts of carboxyl-terminated hyperbranched polyester resin, 3 parts of membrane split nylon fiber, 0.3 part of PVC-g-(MMA-co-AN), 0.3 part of inorganic nano silver.
[0056] The PVC resin used in this embodiment is a PVC resin with a degree of polymerization of 1150-1250 obtained by suspension polymerization, and the length of the film-split nylon fiber used is 7 mm, and the fineness is 1400-1600D. The production method of the medical PVC shoe sole material that the present embodiment can be sterilized by high temperature steam comprises the steps:
[0057] (1) Preparation of composite thermal stabilizer: use the mixed solution of epoxidized soybean oil and bisphenol A diglycidyl ether as auxiliary thermal sta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Fineness | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com