Method for equal molar preparation of lithium iron phosphate by hydrothermal method
A lithium iron phosphate, equimolar technology, applied in the field of lithium-ion battery cathode materials, can solve the problems of raw material cost waste, high reaction impurity content, complicated operation, etc., and achieve the effect of maintaining charge balance and refining lithium iron phosphate particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
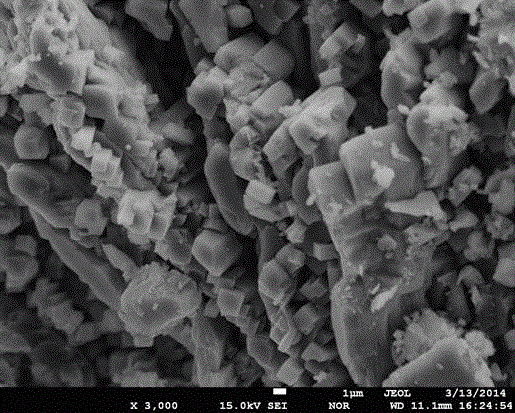
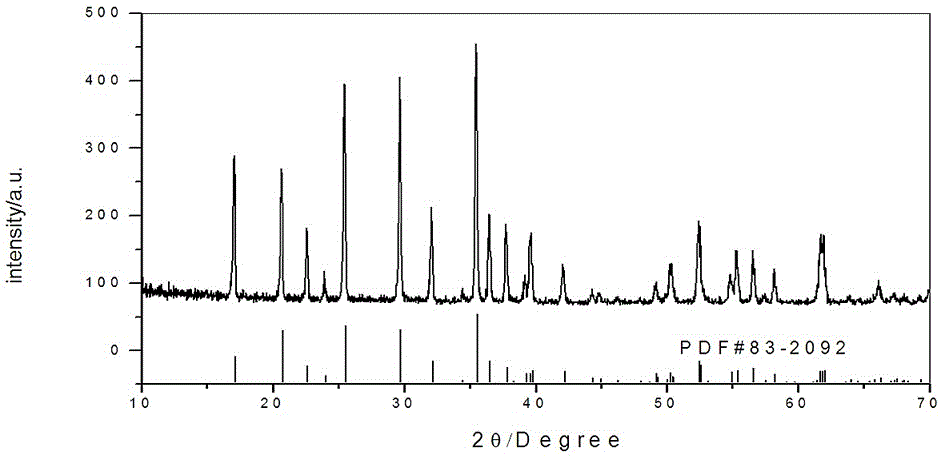
Image
Examples
Embodiment 1
[0025] (1) First weigh 400g of ammonium pyrophosphate and dissolve in 1L of deoxygenated distilled water, and slowly add 1mol of FeSO with electromagnetic stirring 4 ·7H 2 O, heated to 80°C and kept warm for 30min to form mixed solution A;
[0026] (2) Weigh 1molLiH 2 PO 4 , dissolved in 1L deoxygenated distilled water, and stirred to form solution B; LiH 2 PO 4 Weigh the standard as Fe 2+ : Li + =1:1; that is, the ratio of the added substances is the molar ratio Li:Fe:P=1:1:1;
[0027] (3) Mix and stir the mixed solution A and solution B, add it to the high-pressure reactor, and adjust the pH to 6 with 1mol / L ammonia water; feed high-purity nitrogen gas, heat the reactor to 160°C for 6 hours in waves, and cool naturally to At room temperature, filter, wash, dry in a vacuum oven at 80°C for 4 hours, and pulverize to obtain the positive electrode material LiFePO 4 .
[0028] In the prior art, iron ions in the solution easily react with phosphate ions, and agglomerate t...
Embodiment 2
[0032] (1) First weigh 200g of zwitterionic polyacrylamide into 1L of deoxygenated distilled water, and slowly add 1mol of ferrous oxalate with electromagnetic stirring to form a mixed solution A;
[0033] (2) Weigh 1molLiH 2 PO 4 Dissolve in 1L deoxygenated distilled water and stir to form solution B; LiH 2 PO 4 Weigh the standard as Fe 2+ : Li + =1:1; that is, the ratio of the added substances is the molar ratio Li:Fe:P=1:1:1;
[0034] (3) Mix and stir the mixed solution A and solution B, then add it to the high-pressure reactor, adjust the pH to 7 with 1mol / L ammonia water; feed high-purity nitrogen, heat the reactor to 200°C for 4 hours in a wave type, and cool naturally to room temperature, filter, wash, dry in a vacuum oven at 80°C for 4 hours, and pulverize to obtain the positive electrode material LiFePO 4 .
[0035] Wherein the selected iron phosphate stabilizer is zwitterionic polyacrylamide, and the ferrous source is ferrous oxalate. Zwitterionic polyacrylam...
Embodiment 3
[0037] (1) Weigh 200g of soluble starch and dissolve it in 1L of deoxygenated distilled water, heat to 60°C to form gelatinized starch, slowly add 1mol of FeSO with electromagnetic stirring 4 ·7H 2 O, forming a mixed solution A;
[0038] (2) Weigh 1molLiH 2 PO 4 Dissolve in 1L deoxygenated distilled water and stir to form solution B;
[0039] (3) Mix and stir the mixed liquid A and solution B, add it to the high-pressure reactor, and adjust the pH to 8 with ammonia water; feed high-purity argon, heat the reactor to 180°C for 5 hours in a wave manner, cool naturally to room temperature, and filter , washing, drying in a vacuum oven at 80°C for 4 hours, and crushing to obtain the positive electrode material LiFePO 4 .
[0040] Among them, the iron phosphate stabilizer selected is soluble starch. Since the soluble starch is heated and gelatinized and reversed, the starch molecules in the granules stretch and diffuse in all directions, dissolve out of the granules, and the ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com