A class of heterozygous tumor-targeting nanomicelles and uses thereof
A tumor targeting and nanomicelle technology, applied in the application field of tumor targeted therapy, can solve the problems of normal tissue or organ damage, insufficient drug concentration, leakage, etc., and achieve the effect of good safety and stability improvement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
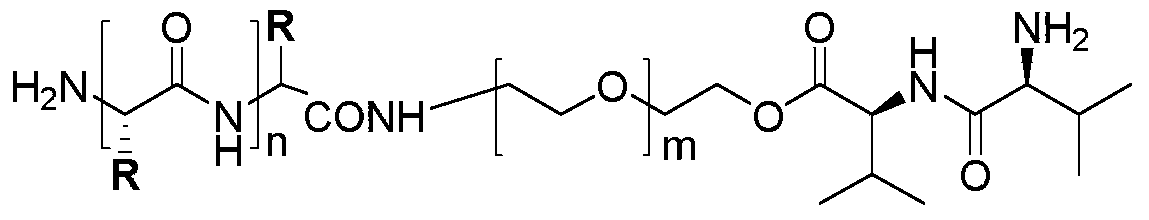
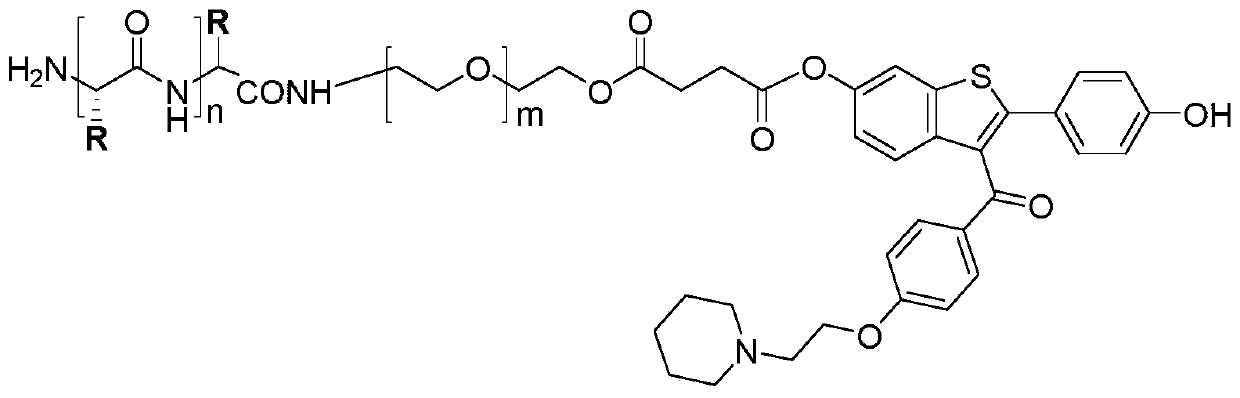
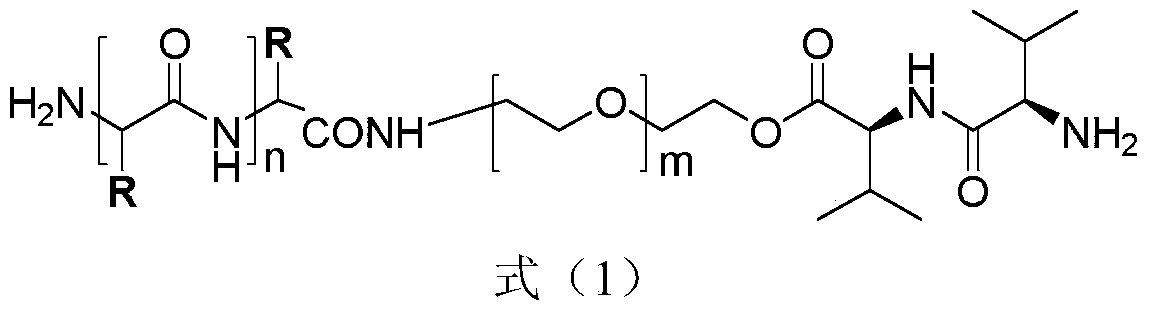
[0029] The preparation method of polymer 1 of the present invention and polymer 2 is shown in reaction formula (I), (II):
[0030]
[0031] Reaction formula (I)
[0032]
[0033] in,
[0034] Reaction formula (II)
[0035] The synthetic method of polymer 1 comprises the following steps:
[0036] Step 1: reacting the azide-protected PEG with the Boc-protected L-valine-L-valine dipeptide in the presence of a condensing agent to obtain a dipeptide-modified PEG intermediate 1;
[0037] Step 2: intermediate 1 is reduced by catalytic hydrogenation to obtain intermediate 2;
[0038] Step 3: Ring-opening polymerization of intermediate 2 with 10-40 equivalents of L-alanine-N-carboxyl-internal anhydride or 2,4-dinitrophenyl protected histidine internal anhydride to obtain intermediate 3 or 4;
[0039] Step 4: Deprotection of intermediates 3 and 4 to obtain polymer 1.
[0040] The synthetic method of polymer 2 comprises the following steps:
[0041] Step 1: Reaction of azido...
Embodiment 1
[0045] Embodiment 1. Preparation of Intermediate 1
[0046]
[0047] At 25°C, Boc-L-Val-L-Val dipeptide (1.2mmol), HO-PEG-N 3 (1mmol), DCC (1mmol) and DMAP (0.1mmol) were added to a 100mL single-necked bottle, and 100mL CH 2 Cl 2 Stir to dissolve, N 2 protection, reacted for 24h, and the reaction solution gradually became turbid. After stopping the reaction, filter, and the filtrate was washed with 5% HCl aqueous solution (50mL×3), and the organic layer was anhydrous Na 2 SO 4 Drying, suction filtration, filtrate concentration, column chromatography (CH 2 Cl 2 :CH 3 OH=10:1) A white waxy solid was obtained with a yield of 56%.
[0048] IR(KBr,cm -1 ):3418(NH,s); 2887(CH 2 CH 2 ,vs); 2105(N 3 , s); 1720, 1669, 1624 (C=O, s); 1112 (CH 2 -O-CH 2 ,vs)
[0049] 1 HNMR (300MHz, CDCl 3 ,δ): 5.25 (NH-CO), 3.99-4.55 (NH-CO); 4.57, 3.97 (NH-C H -CO); 3.64(O-C H 2 C H 2 -O); 2.25, 2.13 (C H (CH 3 ) 2 ); 1.45(C(C H 3 ) 3 );0.93(CH(C H 3 ) 2 )
Embodiment 2
[0050] Embodiment 2. Preparation of Intermediate 2
[0051]
[0052] Intermediate 1 (0.23 mmol) was dissolved in 10 mL CH 2 Cl 2 , then add 20mL of ethanol and 0.05g of 10% Pd / C, hydrogenate at 40°C until the reaction is complete, filter with suction, concentrate the filtrate to 3mL, add dropwise to 50mL of diethyl ether with vigorous stirring, cool and stand in an ice-water bath for 30min, and filter with suction , and dried to obtain a white solid with a yield of 90%.
[0053] IR(KBr,cm -1 ):3412(NH,NH 2 ,s); 2887(CH 2 CH 2 , vs); 1739, 1708, 1676 (C=O, s); 1112 (CH 2 -O-CH 2 ,vs)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com