Preparation method and use method of manganite catalyst applied to catalytic decomposition of nitrogen oxides at low temperature
A technology of nitrogen oxides and low-temperature catalysis, which is applied in the field of pollution control, can solve the problem of low catalytic activity, and achieve the effect of low price and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
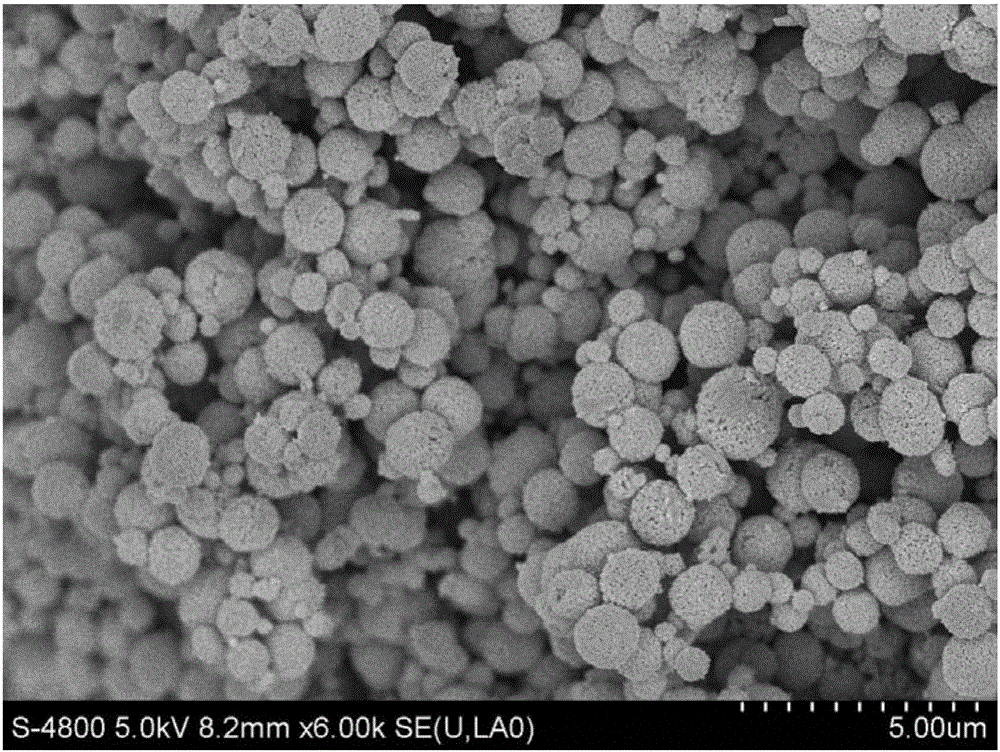
Image
Examples
Embodiment 1
[0020] MnO of the present invention x The -450 catalyst is calcined at 450°C, and the Mn(CH 3 COO) 2 4H 2 O and Na 2 CO 3 The molar ratio is 1:2, the specific preparation method:
[0021] 0.015mol of Mn(CH 3 COO) 2 4H 2 O was added to 90 mL of ethylene glycol, stirred and heated to 120 ° C, when the temperature reached 120 ° C, nitrogen gas was injected, and then 0.2 mol / L Na was injected at a flow rate of 4 mL / min. 2 CO 3 150mL solution, and the mixed solution was stirred vigorously, and the heating was stopped after 1 hour of reaction to obtain the mixed solution. When the temperature of the mixed solution dropped below 50°C, the mixed solution was suction-filtered, and washed three times with ethanol and deionized water, and the filtered After the final sediment was dried at 90°C for 8 hours, it was put into a vacuum drying oven for further drying at 50°C for 8 hours, and finally calcined at 450°C for 4 hours. It was tabletted, crushed, and sieved with 40-60 meshes...
Embodiment 2
[0024] The calcination temperature of the catalyst described in Example 1 is changed from 450°C to 500°C, and the specific preparation method is:
[0025] 0.015mol of Mn(CH 3 COO) 2 4H 2 O was added to 90 mL of ethylene glycol, stirred and heated to 120°C. When the temperature reached 120°C, nitrogen gas was introduced, and then 0.2mol / L Na was injected at a flow rate of 4mL / min. 2 CO 3 150mL solution, and the mixed solution was stirred vigorously, and the heating was stopped after 1 hour of reaction to obtain the mixed solution. When the temperature of the mixed solution dropped below 50°C, the mixed solution was suction-filtered, and washed three times with ethanol and deionized water, and the filtered After drying the final sediment at 90°C for 8 hours, put it into a vacuum drying oven for further drying at 50°C for 8 hours, and finally calcined at 500°C for 4 hours, and obtained low-temperature catalytic decomposition Manganese oxide catalyst for nitrogen oxides, denot...
Embodiment 3
[0028] Mn(CH 3 COO) 2 4H 2 O was added to ethylene glycol and stirred and heated to 120°C. When the temperature reached 120°C, nitrogen gas was introduced, and then 0.2mol / L Na was injected at a flow rate of 3mL / min. 2 CO 3 solution, and stir the mixed solution vigorously, and stop heating after 1 hour of reaction to obtain the mixed solution. When the temperature of the mixed solution drops below 50°C, the mixed solution is suction-filtered, and washed with ethanol and deionized water repeatedly for 5 times, and the filtered After the sediment was dried at 120°C for 8 hours, it was put into a vacuum drying oven for further drying at 50°C for 10 hours, and finally calcined at 470°C for 5 hours. After tableting, crushing, and sieving with 40-80 meshes, low-temperature catalytic decomposition of nitrogen was obtained. Oxides of manganese oxide catalysts. Among them, Mn(CH 3 COO) 2 4H 2 The molar ratio of O to ethylene glycol is 1:110, (Mn(CH 3 COO) 2 4H 2 O and Na 2 C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com