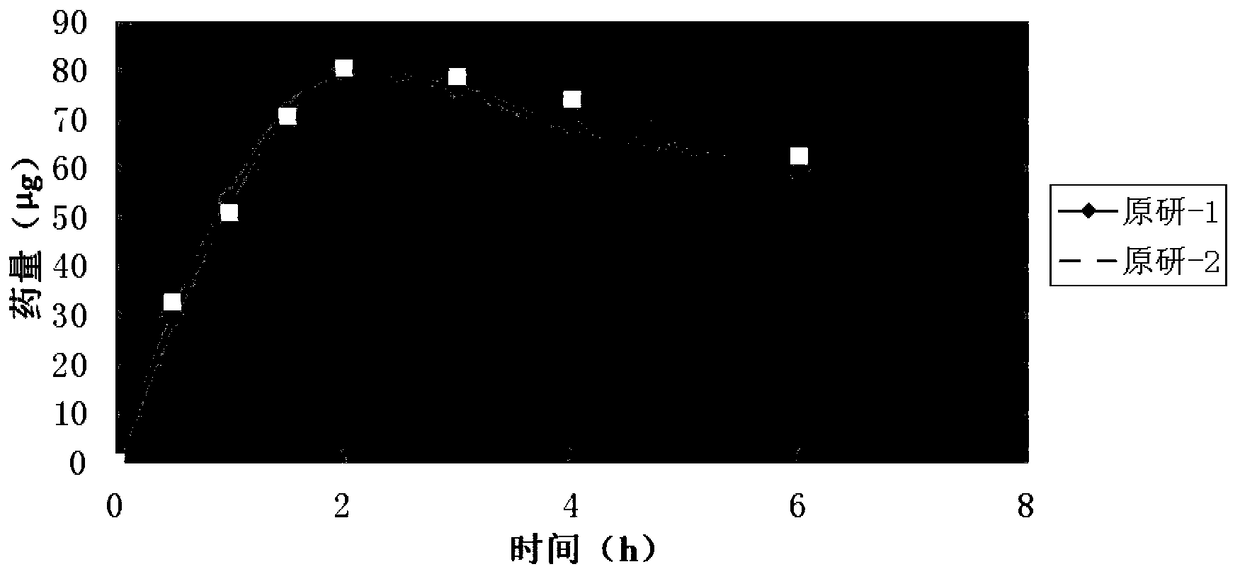
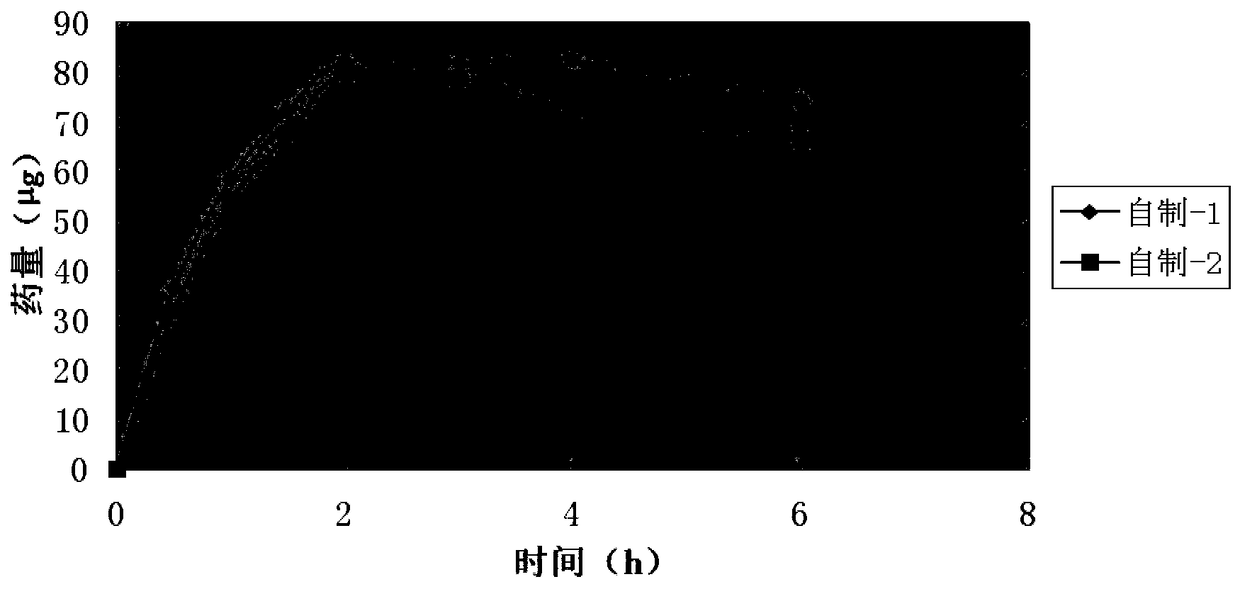
A kind of tobramycin dexamethasone nano-suspension eye drops and preparation method thereof
A technology of dexamethasone and nano-suspension, which is applied in the fields of pharmaceutical formulation, liquid transportation, emulsion transportation, etc., can solve the problems of insufficient sterilization and special requirements for sterilization equipment, and achieve stable and reliable product quality, sterilization The method is simple and effective, and the eye area is comfortable and safe to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] prescription:
[0032]
[0033] Preparation Process:
[0034] 1) Weigh the prescribed amount of tobramycin, sodium chloride, and edetate disodium, dissolve them in an appropriate amount of water for injection, measure benzalkonium bromide solution, and add Tween 80 solution to the above solution, stir evenly, 0.22 μm Sterilize by filtering through a pore filter; weigh the prescribed amount of hypromellose (E50), dissolve it in an appropriate amount of water for injection, filter and sterilize with a 0.22 μm microporous membrane, mix with the above solution, and set aside.
[0035] 2) Weigh the prescribed amount of dexamethasone, suspend it in an appropriate amount of water for injection, add dilute hydrochloric acid to adjust the pH value to 3.0-4.0, and shear it with a high-shear dispersing emulsifier for 15 minutes (1000r / min) to prepare the mixed dexamethasone Suspension (particle size range: 50-200nm), sterilized by filtration through a 0.22 μm microporous membr...
Embodiment 2
[0038] prescription:
[0039]
[0040] Preparation Process:
[0041] 1) Weigh the prescribed amount of tobramycin, sodium chloride, and edetate disodium, dissolve them in an appropriate amount of water for injection, measure benzalkonium bromide solution, and add Tween 80 solution to the above solution, stir evenly, 0.22 μm Sterilize by filtering through a pore filter; weigh the prescribed amount of hypromellose (15000 cps), dissolve it in an appropriate amount of water for injection, filter and sterilize with a 0.22 μm microporous filter, mix with the above solution, and set aside.
[0042] 2) Weigh the prescribed amount of dexamethasone, suspend it in an appropriate amount of water for injection, add dilute hydrochloric acid to adjust the pH value to 3.0-4.0, and shear it with a high-shear dispersing emulsifier for 15 minutes (1000r / min) to prepare the mixed dexamethasone Suspension (particle size range: 50-200nm), sterilized by filtration through a 0.22 μm microporous m...
Embodiment 3
[0045] prescription:
[0046]
[0047]
[0048] Preparation Process:
[0049] 1) Weigh the prescribed amount of tobramycin, sodium chloride, and edetate disodium, dissolve them in an appropriate amount of water for injection, measure benzalkonium bromide solution, and add Tween 80 solution to the above solution, stir evenly, 0.22 μm Sterilize by filtering through a pore filter; weigh the prescribed amount of hypromellose (K100M), dissolve it in an appropriate amount of water for injection, filter through a 0.22 μm microporous membrane, mix with the above solution, and set aside.
[0050] 2) Weigh the prescribed amount of dexamethasone, suspend it in an appropriate amount of water for injection, add dilute hydrochloric acid to adjust the pH value to 3.0-4.0, and shear it with a high-shear dispersing emulsifier for 15 minutes (1000r / min) to prepare the mixed dexamethasone Suspension (particle size range: 50-200nm), sterilized by filtration through a 0.22 μm microporous me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com