Preparation method of triketone compound and triketone compound intermediate
A technology of compounds and triketones, which is applied in the field of preparation of triketone compounds and their intermediates, can solve problems such as unfavorable industrial production, and achieve the effects of high product quality, simple process, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
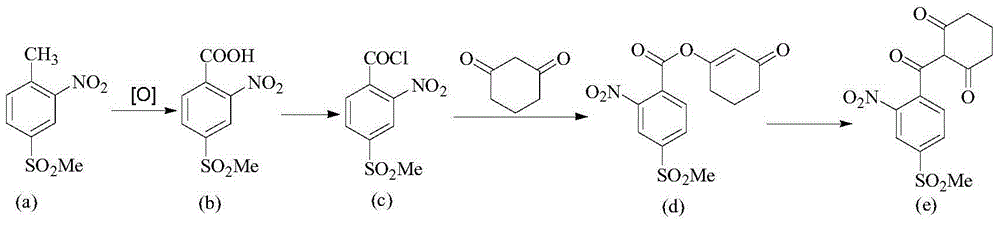
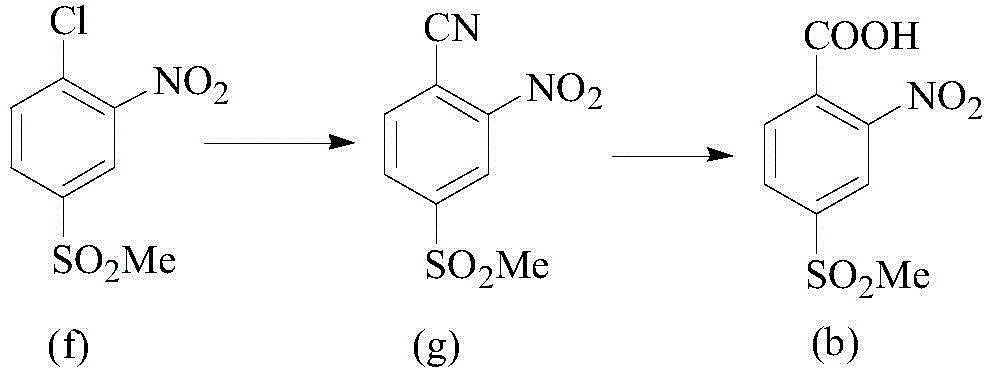
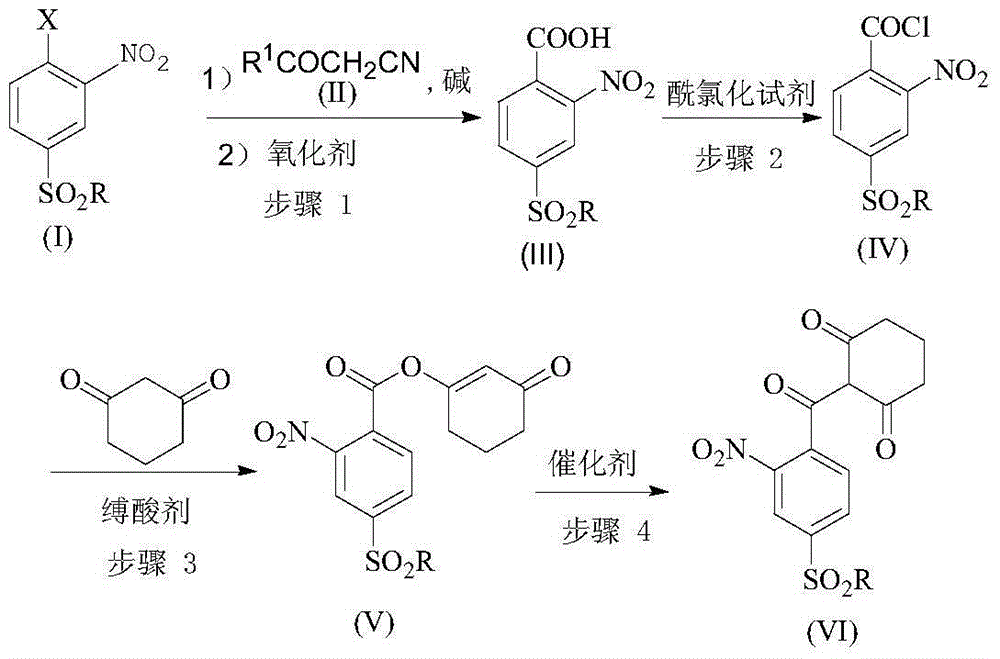
[0033] A preparation method for triketones, comprising the steps of:
[0034] Step 1, in a solvent and in the presence of a base, react compound (I) and compound (II) at a certain temperature, and then continue to react with an oxidant to obtain compound (III);
[0035] Step 2, compound (III) is reacted with an acid chloride reagent to obtain compound (IV);
[0036] Step 3, under the action of an acid-binding agent, compound (IV) undergoes an esterification reaction with 1,3-cyclohexanedione to obtain compound (V);
[0037] Step 4, under the action of the catalyst, the compound (V) is rearranged to obtain the triketone target compound (VI);
[0038] The reaction formula is expressed as follows:
[0039]
[0040] Wherein, X is halogen, sulfonyl or sulfinyl, R is C1-C6 alkyl or C6-C10 aryl, R 1 is alkoxy, amino, alkylamino, alkyl, aryl or hydrogen.
[0041] Said X is preferably fluorine, chlorine or bromine; said R is preferably methyl; said R 1It is preferably methoxy, ...
Embodiment 1
[0049] Embodiment 1: Preparation of 2-nitro-4-methylsulfonylbenzoic acid
[0050] In a 1000mL three-necked flask, add 400mL DMSO, 53g sodium hydroxide, 66.4g methyl cyanoacetate and 150g 2-nitro-4-methylsulfonylchlorobenzene, control the temperature of the reactant within 60°C, and stir for 1 hour. Then add 124g 35% H 2 o 2 , reacted for 2 hours, quenched hydrogen peroxide, distilled and recovered DMSO, added water, acidified with hydrochloric acid, solid precipitated, filtered, and the filter cake was dried to obtain 148.6g of 2-nitro-4-methylsulfonylbenzoic acid. 1 H-NMRδppm (DMSO-d6) 14.42 (br, 1H), 8.53 (d, J = 1.6Hz, 1H), 8.33 (dd, J1 = 1.6Hz J2 = 8.0Hz, 1H), 8.12 (d, J = 8.0Hz , 1H), 3.39(s, 3H).
Embodiment 2
[0051] Embodiment 2: Preparation of 2-nitro-4-methylsulfonylbenzoic acid
[0052] In a 1000mL three-necked flask, add 400mL NMP, 128g triethylamine, 55g cyanoacetamide and 149g 2-nitro-4-trifluoromethylchlorobenzene, control the temperature of the reactant at 30°C, and stir for 2 hours. Then add 162g 40% H 2 o 2 , reacted for 2 hours, quenched hydrogen peroxide, recovered NMP by distillation, added water, acidified with hydrochloric acid, solid precipitated, filtered, and the filter cake was dried to obtain 151g of 2-nitro-4-methylsulfonylbenzoic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com