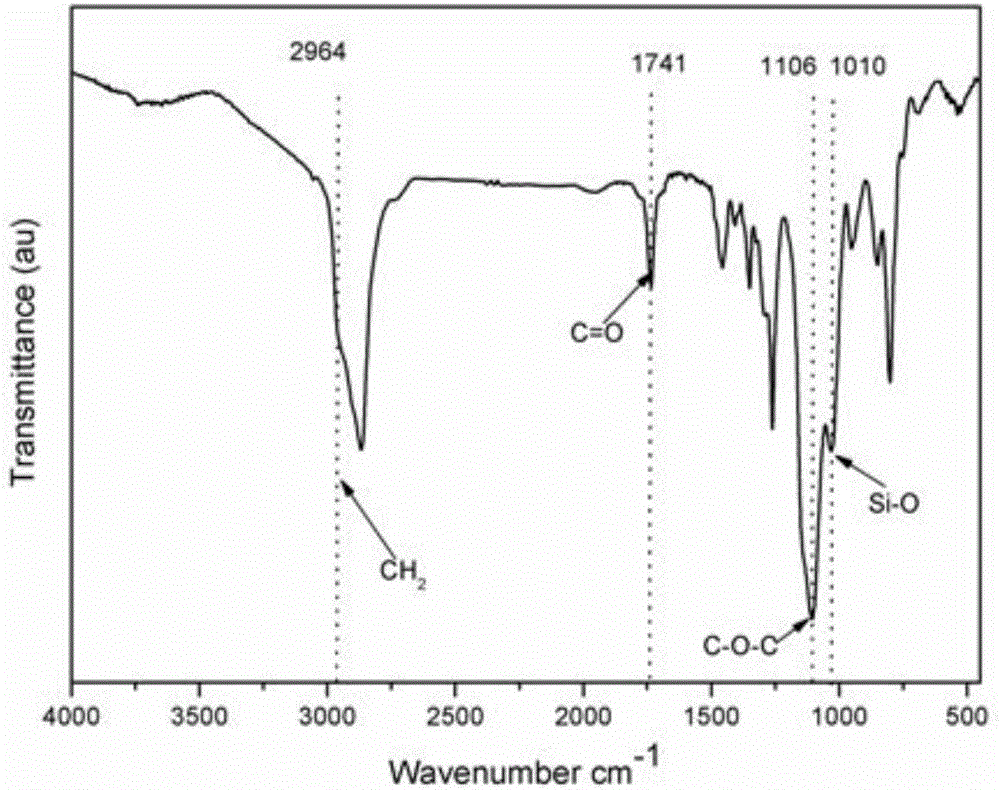
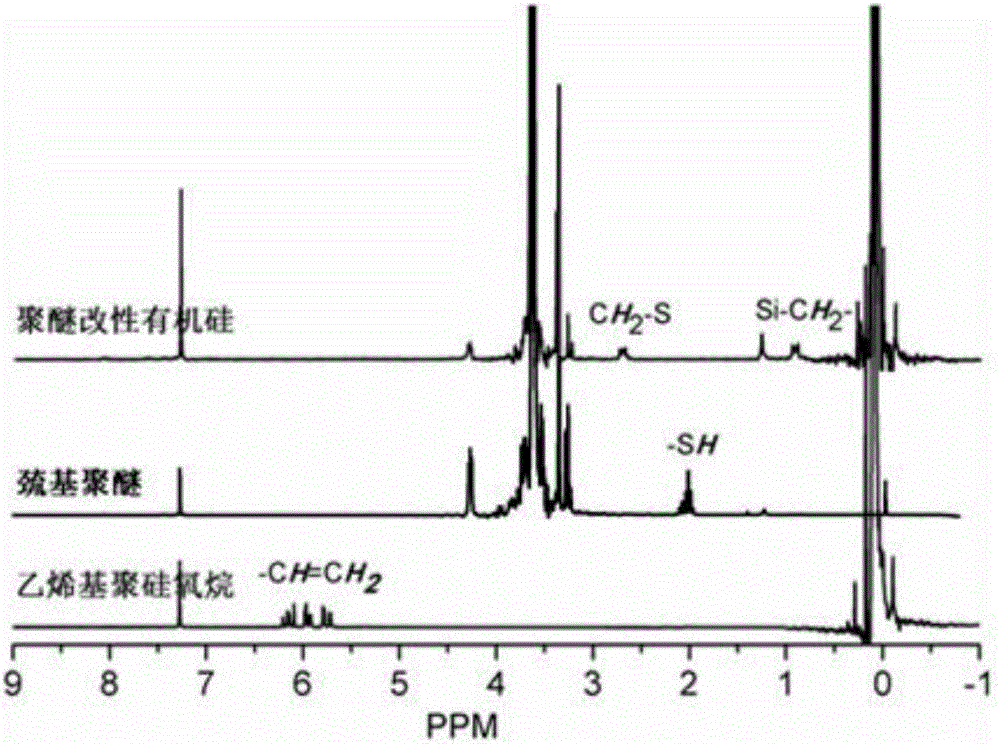
Organosilicon surfactant and thiol-ene click chemistry process preparation method thereof
A surfactant and silicone technology, applied in chemical instruments and methods, chemical/physical processes, transportation and packaging, etc., can solve problems such as long reaction time, affecting product performance, harsh reaction conditions, etc., to achieve short reaction time, The effect of reducing the preparation cost and satisfying the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] (1) Preparation of mercapto polyether
[0032] The synthetic route of mercapto polyether is as follows:
[0033]
[0034] Dissolve 10-30g of capped polyether and 4.6-13.8g of thioglycolic acid in 150-500mL of toluene, add it to a 1000mL single-necked flask, and then place it in an oil bath with a magnetic heating stirrer to undergo azeotropic distillation for 6- 24h. After the reaction, the solvent was removed under reduced pressure, and the residual solution was dissolved in dichloromethane and precipitated in cold ether. The operation was repeated three times, and vacuum-dried at room temperature for 6-24 hours to obtain mercaptopolyether. Or firstly halogenate the end-capped polyether, undergo a nucleophilic substitution reaction with sodium hydrosulfide, adjust the pH value of the solution to 5-6.5, precipitate in cold ether three times, and vacuum-dry at room temperature for 6-24 hours to obtain 8-24 g of mercaptopolyether;
[0035] (2) Preparation of vinyl po...
Embodiment 1
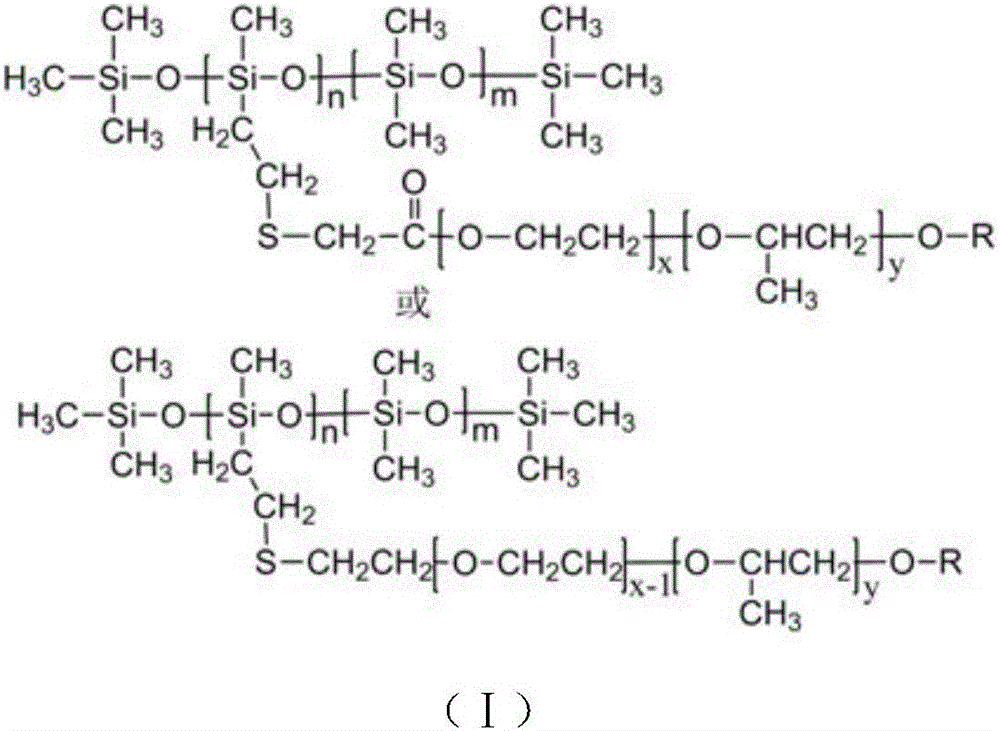
[0043] A kind of organosilicon surfactant, this surfactant has structure shown in formula (I):
[0044]
[0045] Among them, m and n are both integers and 201~10 straight-chain or isomeric long-chain alkyl, or alkylphenyl.
Embodiment 2
[0047] A kind of mercapto-alkene click chemistry method preparation method of organosilicon surfactant, concrete steps are as follows:
[0048] (1) Preparation of mercapto polyether
[0049] Dissolve 10-30g of capped polyether and 4.6-13.8g of mercaptoacetic acid in 150-500mL of toluene, add it to a 1000mL single-necked flask, and then place it in an oil bath with a magnetic heating stirrer for azeotropic distillation for 6-24 hours. After the reaction, the solvent was removed under reduced pressure, and the residual solution was dissolved in dichloromethane and precipitated in cold ether. Repeat the operation three times, and vacuum-dry at room temperature for 6-24 hours to obtain 8-24 g of mercaptopolyether;
[0050] (2) Preparation of vinyl polysiloxane
[0051] Add 10-30g of octamethylcyclotetrasiloxane, 5-25g of tetramethyltetravinylcyclotetrasiloxane, and 0.5-1.5g of hexamethyldisiloxane into a 250mL three-necked flask under the protection of inert gas. Place it in an ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com