Synthesis and organic luminescent device of bisphenothiazine dioxide derivative
A bisphenothiazine and dioxide technology, which is applied in the fields of luminescent materials, organic chemistry, electric solid devices, etc., can solve the problems of increasing the cost of device fabrication, and achieve high glass transition temperature and decomposition temperature, and good thermal stability. , Improve the effect of luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
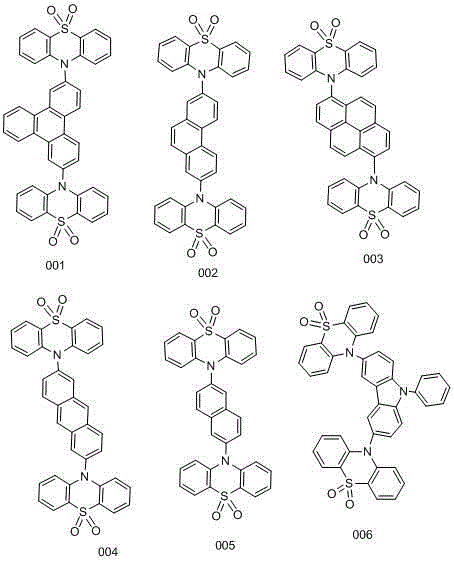
[0048] Add 48.30mmol of phenothiazine and 48.49mmol of sodium tert-butoxide into the reaction flask, then add 33ml of toluene, under nitrogen protection, stir for 10min, then add 0.41mmol of tris(dibenzylideneacetone)dipalladium, tri-tert-butylphosphine 2.45mmol, 2,7-dibromotriphenylene 16.20mmol, gas exchanged three times, reflux at 110°C overnight. After the reaction is finished, post-treatment, extraction with 100ml of dichloromethane, after evaporating the organic phase, a small amount of dichloromethane dissolves and precipitates the product with petroleum ether. The precipitated product is washed 3 times with 100ml of petroleum ether, and washed with 100ml of ethanol for 2 times to obtain a light yellow color The product is 14.09 mmol, and the yield is 87.00%.
[0049] Add 14.00 mmol of 2,7-bis(10H-phenothiazin-10-yl) triphenylene and dichloromethane into the reaction flask, slowly add 35.00 mmol of m-chloroperoxybenzoic acid in batches under ice cooling, and then After...
Embodiment 2
[0052] Add 48.60mmol of phenothiazine and 48.49mmol of sodium tert-butoxide into the reaction flask, then add 33ml of toluene, under nitrogen protection, stir for 10min, then add 0.203mmol of tris(dibenzylideneacetone)dipalladium, tri-tert-butylphosphine 1.295mmol, 16.20mmol of 2,7-dibromophenanthrene, three times of ventilation, 110 ℃ reflux overnight. After the reaction is finished, post-treatment, extraction with 100ml of dichloromethane, after evaporating the organic phase, a small amount of dichloromethane dissolves and precipitates the product with petroleum ether. The precipitated product is washed 3 times with 100ml of petroleum ether, and washed with 100ml of ethanol for 2 times to obtain a light yellow color The product is 14.01 mmol, and the yield is 86.48%.
[0053] Add 14.00 mmol of 2,7-bis(10H-phenothiazin-10-yl)phenanthrene and dichloromethane into the reaction flask, slowly add 35.00 mmol of m-chloroperoxybenzoic acid in batches under ice bath, and then react i...
Embodiment 3
[0056] Add 48.62mmol of phenothiazine and 48.49mmol of sodium tert-butoxide into the reaction flask, then add 33ml of toluene, under nitrogen protection, stir for 10min, then add 0.203mmol of tris(dibenzylideneacetone)dipalladium, tri-tert-butylphosphine 1.295mmol, 16.20mmol of 2,7-dibromopyrene, three ventilations, reflux at 110°C overnight. After the reaction is finished, post-treatment, extraction with 100ml of dichloromethane, after evaporating the organic phase, a small amount of dichloromethane dissolves and precipitates the product with petroleum ether. The precipitated product is washed 3 times with 100ml of petroleum ether, and washed with 100ml of ethanol for 2 times to obtain a light yellow color The product is 14.03 mmol, and the yield is 87.58%.
[0057] Add 14.00mmol of 2,7-bis(10H-phenothiazin-10-yl)pyrene and dichloromethane into the reaction flask, slowly add 35.00mmol of m-chloroperoxybenzoic acid in batches under ice bath, and then react in ice bath for 30mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| luminous efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com