GAG (glycosaminoglycan) analogue realizing cell membrane modification, synthetic method of GAG analogue and application method of GAG analogue in in-vitro induced stem cell directional differentiation
A technology of glycosaminoglycans and synthesis methods, which is applied in the field of glycosaminoglycans labeled with green fluorescein and can solve the problems of uncontrollable sulfonation degree, limited wide application, complex synthesis and purification process, etc., and achieves The synthesis method and purification process are simple, the degree of sulfonation is easy to control, and the effect of strong universality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] A kind of synthetic method of the sugar amine group analog of phospholipid group anchoring, comprises the following steps:
[0045] (1) Using RAFT technology to prepare a copolymer of SS, MAG and FluMA three monomers (molecular weight is in the range of 6000-10000), and using the chemical reaction between active groups to synthesize MAL-DPPE and DPPE-pSMF;
[0046](2) Dissolving the prepared DPPE-pSMF in a serum-free culture solution at a certain concentration, and incubating the Hela cells for a certain period of time in a 37°C constant temperature incubator, and then studying the modification of the cell membrane surface;
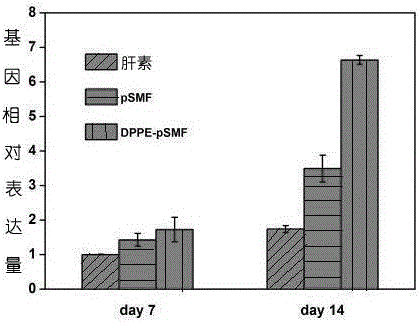
[0047] (3) Dissolve DPPE-pSMF in serum-free culture medium, incubate ESCs in a constant temperature incubator at 37°C for a certain period of time, add fresh neural differentiation induction medium after washing, and study how it promotes the directional differentiation of ESCs into neurons after several days Case.
[0048] The principle of the pr...
Embodiment 1
[0054] (1) Select SS, MAG, FluMA as monomers, in DMF / H 2 RAFT polymerization is carried out in a mixed solvent of O (V / V=1:1). After a certain period of reaction, the reaction solution is dialyzed and purified to obtain a terpolymer pSMF, which is added to MAL-DPPE to obtain a phospholipid group. Anchored glycosaminoglycan analog DPPE-pSMF;
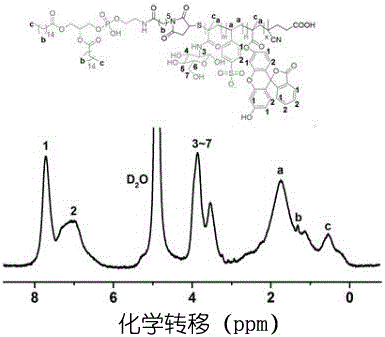
[0055] (2) Characterize the obtained substance by proton nuclear magnetic resonance spectrum and infrared spectrum.
[0056] The result is as figure 1 As shown, the phospholipid-anchored glycosaminoglycan analog DPPE-pSMF 1 H-NMR spectrum.
[0057] The phospholipid-anchored glycosaminoglycan analogs are used to modify the cell membrane of the model cells, including the following steps: (1) the model cells Hela are planted on the cell climbing plate, and a certain concentration of phospholipid-anchored glycosaminoglycans is prepared. Serum-free medium of analogs, incubate Hela cells at 37°C for a certain period of time;
[0058] (2) W...
Embodiment 2
[0060] (1) Plant Hela cells on the cell slide plate, add serum-free high-glucose DMEM culture medium containing 3.4 μM terpolymer pSMF and phospholipid group-anchored polymer DPPE-PSMF respectively, and incubate at 37°C Incubate in the box for 1 h, and the cells incubated in serum-free high-glucose DMEM without any polymer were used as the control group;
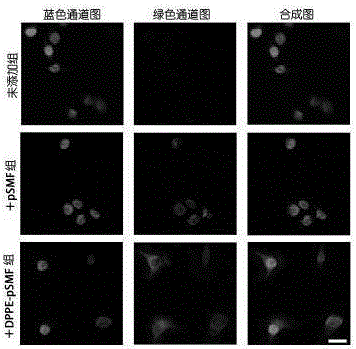
[0061] (2) Discard the above-mentioned culture solution for incubating cells, wash the cells with sterile PBS 2 to 3 times to remove excess polymers, fix the cells on the slide plate with 4% paraformaldehyde solution, and use DAPI reagent to carry out cell nuclei After staining, the modification of the phospholipid-anchored polymer on the surface of the cell membrane was observed under a confocal fluorescence microscope.
[0062] The result is as figure 2 Shown, Hela cells were incubated with pSMF and DPPE-pSMF in the culture solution environment without adding polymer for 1 h, the situation of polymer modification on the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com