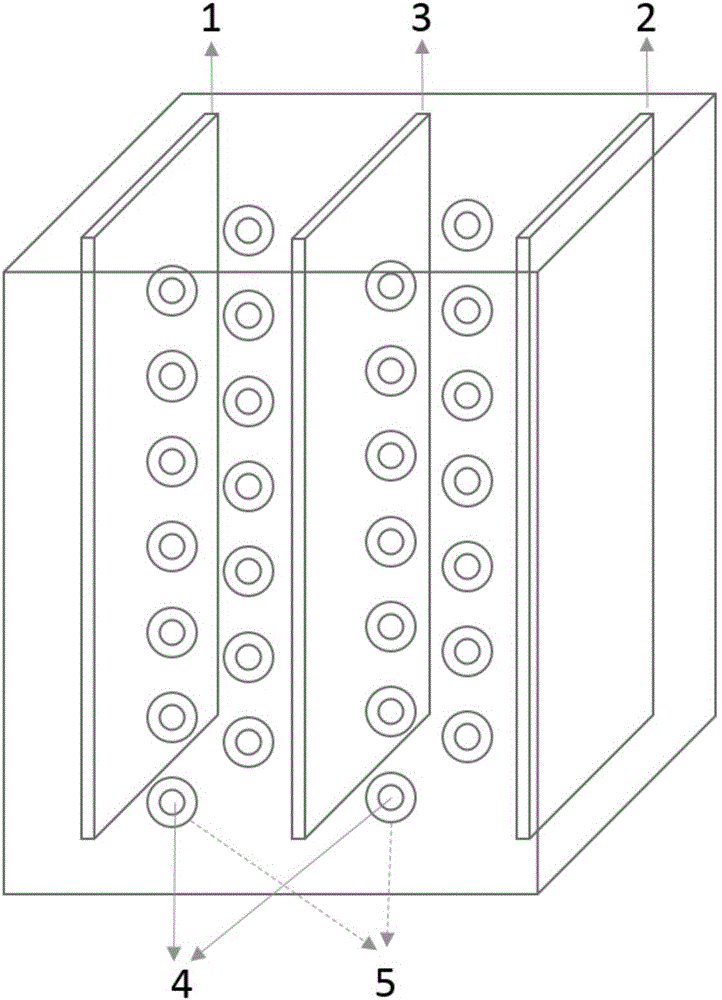
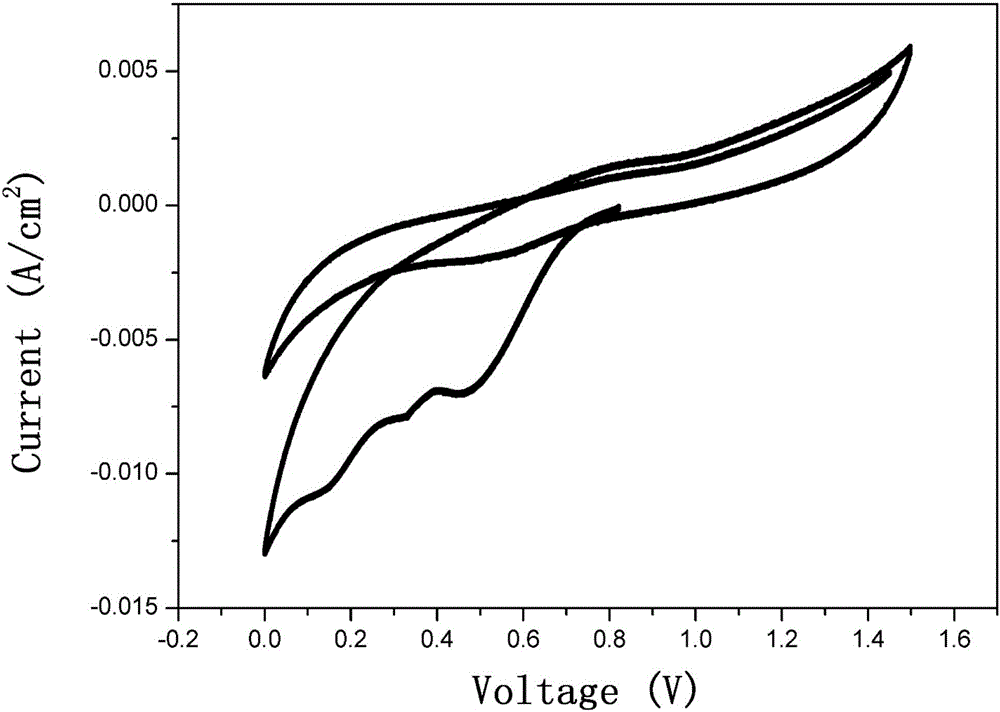
Organic zinc ion secondary battery
A secondary battery and zinc ion technology, applied in secondary batteries, battery electrodes, circuits, etc., can solve the problems of battery flatulence, easy hydrogen evolution, battery short circuit, etc., and achieve high power density, high capacity density, and excellent cycle stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0041] Example 1. Stir 7g of black phosphorus, 1.5g of acetylene black and 1.5g of polytetrafluoroethylene and coat it on copper foil, cut it into electrode sheets of a certain size and dry them in vacuum to constant weight. Use this electrode sheet as the positive electrode and the negative electrode. Extremely 25 micron thick zinc foil;
[0042] Then dissolve zinc sulfate and sodium dodecylbenzenesulfonate in N,N-dimethylformamide to form a 0.01mol / L solution, and use this solution as an electrolyte to assemble a zinc ion secondary battery.
example 2
[0043] Example 2. Stir 7g of phosphorene, 1.5g of graphite and 1.5g of polytetrafluoroethylene and coat it on copper foil, cut it into electrode sheets of a certain size and vacuum-dry them to constant weight. Use this electrode sheet as the positive electrode and the negative electrode Extremely 50 micron thick zinc foil;
[0044] Then dissolve zinc chloride and polyethylene glycol-polypropylene glycol-polyethylene glycol triblock copolymer in propylene carbonate to form a 0.01mol / L solution, and use this solution as an electrolyte to assemble zinc ions secondary battery.
example 3
[0045] Example 3, the composite of 7g black phosphorus and graphene, 2g carbon nanotubes and 1g carboxymethyl cellulose are stirred and coated with copper foil, cut into electrode sheets of a certain size and vacuum-dried to constant weight. The electrode sheet is used as the positive electrode, and the negative electrode is a composite of 7g zinc and 3g graphite; then zinc acetate is dissolved in a mixed solution of propylene carbonate and N,N-dimethylformamide with a volume ratio of 2:1 to form a 0.01mol / L solution, and this solution is used as electrolyte, and then assembled into a zinc ion secondary battery.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com