Biodegradable medicine hydrogel and preparation method and application thereof
A biodegradable and hydrogel technology, applied in the fields of application, medical science, surgery, etc., can solve the problems that cannot meet the requirements of gelation speed and degradation time, etc., and achieve the effect of controllable performance, fast speed and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0087] 2. Preparation and use of medical hydrogel
[0088] Method 1: a method for preparing the above-mentioned medical hydrogel by in-situ cross-linking forming hydrogel, which is realized by changing the pH value of the corresponding buffer solution.
[0089] The specific method is to dissolve the first component containing nucleophilic functional groups in buffer A with a pH of 7.5-12.5 (preferably 9.5-10.2) to obtain solution A, and dissolve the second component containing electrophilic functional groups in pH In the buffer B of 2.0-6.5 (preferably 3.9-6.0), the solution B is obtained, and then the solution A and the solution B are mixed, and the first component and the second component undergo a cross-linking reaction to form a hydrogel.
[0090]Method 2: Another method for preparing the above-mentioned medical hydrogel by in-situ cross-linking forming hydrogel is to mix the first component containing the nucleophilic functional group and the second component containing t...
Embodiment 1
[0137] Embodiment 1, prepare hydrogel with single nucleophilic component and double nucleophilic component
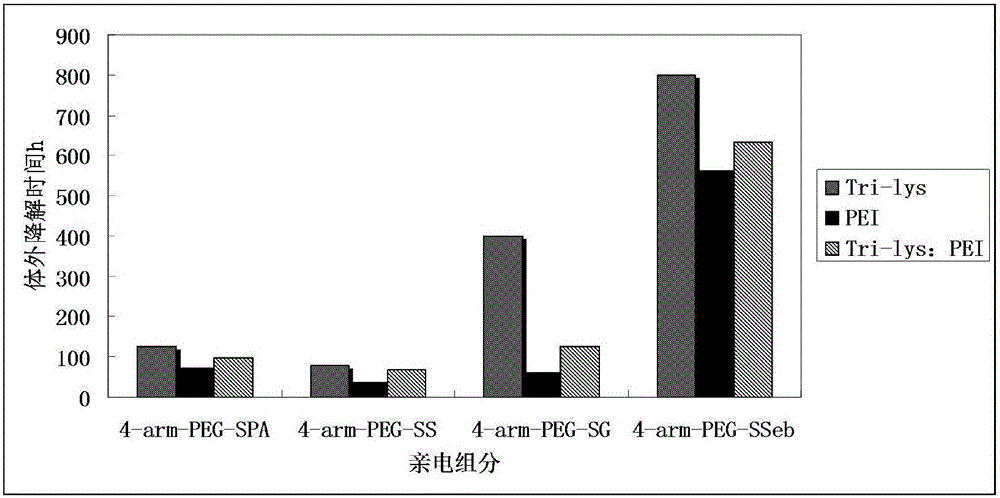
[0138] In order to compare the performance of hydrogels prepared with a single nucleophilic (or electrophilic) component and a double nucleophilic (or electrophilic) component, this example uses a nucleophilic component (the first component) hyperbranched polymer Ethyleneimine (PEI-1800, molecular weight 1800, structural repeating unit see Figure 7B , the primary amino group content is 35%, purchased from Aladdin Reagent Company) and trilysine (Tri-lys, purchased from Shanghai Gil Biochemical Reagent Company) as examples, compare the two alone with the electrophilic component (the second group points) Four-arm N-hydroxysuccinimide propionate-based polyethylene glycol (4-arm-PEG-SPA, molecular weight 10000), four-arm N-hydroxysuccinimide-succinate-based polyethylene glycol Diol (4-arm-PEG-SS, molecular weight 10000), four-arm N-hydroxysuccinimide glutarate-based polyeth...
Embodiment 2
[0149] Embodiment 2, changing the proportion of nucleophilic components to prepare hydrogel
[0150] The first component whose end group is a nucleophilic functional group is hyperbranched polyethyleneimine 1800 (PEI-1800, structural repeating unit see Figure 7B , molecular weight 1800, primary amino group content is 35%, purchased from Aladdin Reagent Company), and / or four-arm mercapto polyethylene glycol (4-arm-PEG-SH, molecular weight is 10000), and / or trilysine acid (Tri-lys, Shanghai Gil Biochemical Reagent Co.), and / or trilysine hydrochloride (Tri-lys-HCl), and / or two-arm aminopolyethylene glycol (2-arm-PEG-NH 2, the molecular weight is 10000), the second component whose end group is an electrophilic functional group is four-arm N-hydroxysuccinimide glutarate-based polyethylene glycol (4-arm-PEG-SG, molecular weight 10000), The ratio of the nucleophilic components was changed (see Table 2 for the formula) to prepare the hydrogel and detect its performance. The preparati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Strength | aaaaa | aaaaa |
| Strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com