Dinuclear complex catalyst, preparation method and application thereof in preparing hydrogen by catalyzing formic acid decomposition
A technology for binuclear complexes and catalysts, applied in the field of binuclear complex catalysts and their preparation, can solve the problems of complex preparation process and high catalyst cost, and achieve the effects of simple preparation process, good solubility and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
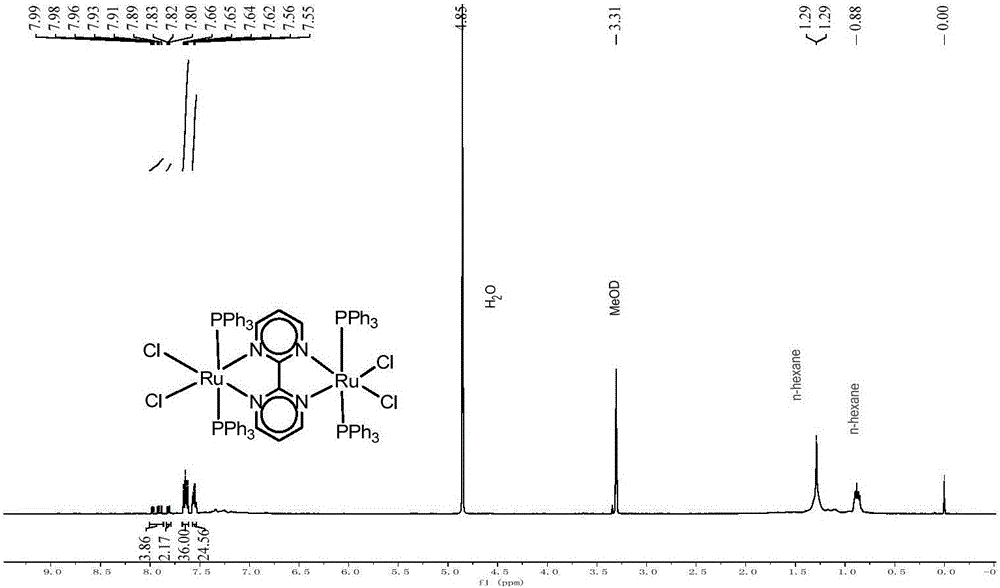
[0026] Ru 2 Cl 2 (PPh 3 ) 4 Preparation of (μ-biim) and Catalysis of HCO 2 H decomposes to produce hydrogen:
[0027] (1) RuCl 2 (PPh 3 ) 3 (96.6mg, 100.0μmol)) and bidiimidazole (14.2mg, 106.0μmol) were added to CH with a volume ratio of 1:1 2 Cl 2 / CH 3 OH solution (20mL), under nitrogen protection, stirred at 25°C for 3h; after the reaction, the solution was evaporated in vacuo to remove the solvent, washed with n-hexane, filtered under reduced pressure, the solid was collected and dried in vacuo to give dark green solid RuCl 2 (H 2 biim) (PPh 3 ) 2 Product 53.6mg, yield 65%;
[0028] (2) RuCl 2 (H 2 biim) (PPh 3 ) 2 (53.6mg, 64.5μmol) by adding CH at a volume ratio of 1:1 2 Cl 2 / CH 3 In OH solution (20mL), KOH solid (3.6mg, 64.5μmol) was added thereto, under the protection of nitrogen, stirred at 25°C for 2h; use CH 2 Cl 2 (10mL) was dissolved, filtered under reduced pressure, the filtrate was evaporated under reduced pressure to remove the solvent,...
Embodiment 2
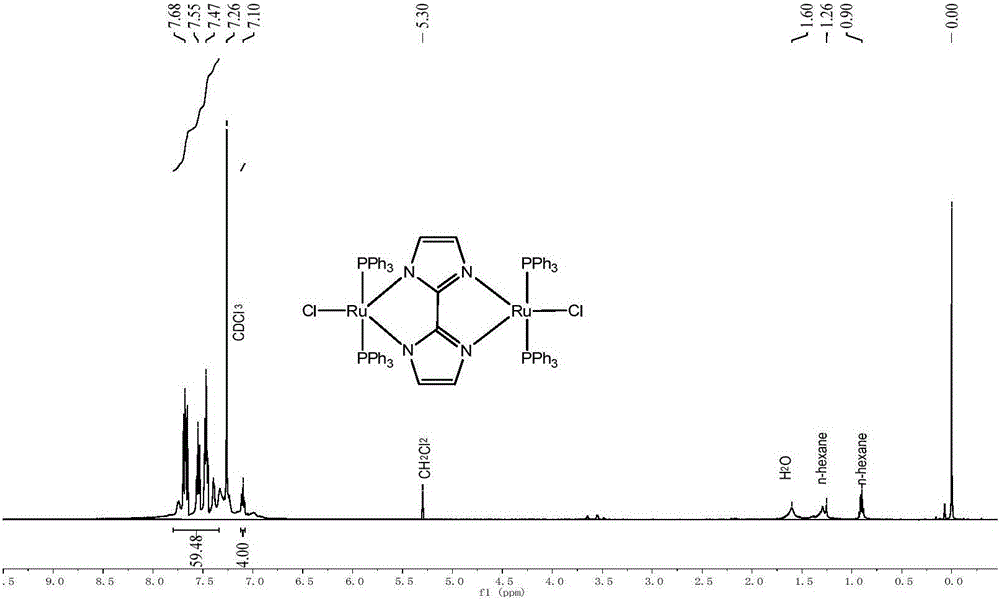
[0033] Ru 2 Cl 4 (PPh 3 ) 4 Preparation of (μ-bpym) and Catalytic Decomposition of Formic Acid to Hydrogen Production:
[0034] (1) Weigh RuCl 2 (PPh 3 ) 3 Add (104.0mg, 108.5μmol) and bipyrimidine (8.6mg, 54.2μmol) into 20mL of anhydrous methanol; vacuumize with an oil pump, fill in nitrogen, and repeat this gas replacement step three times. Under the protection of nitrogen, stir and react at 20°C for 3 hours, and the solution turns dark green; after the reaction, cool to room temperature, filter under reduced pressure, and collect the solid; wash the solid with ether, filter under reduced pressure, collect the solid, and dry in vacuum to obtain a brick red color Solid Ru 2 Cl 4 (PPh 3 ) 4 (μ-bpym) product 56.4 mg, yield 67%.
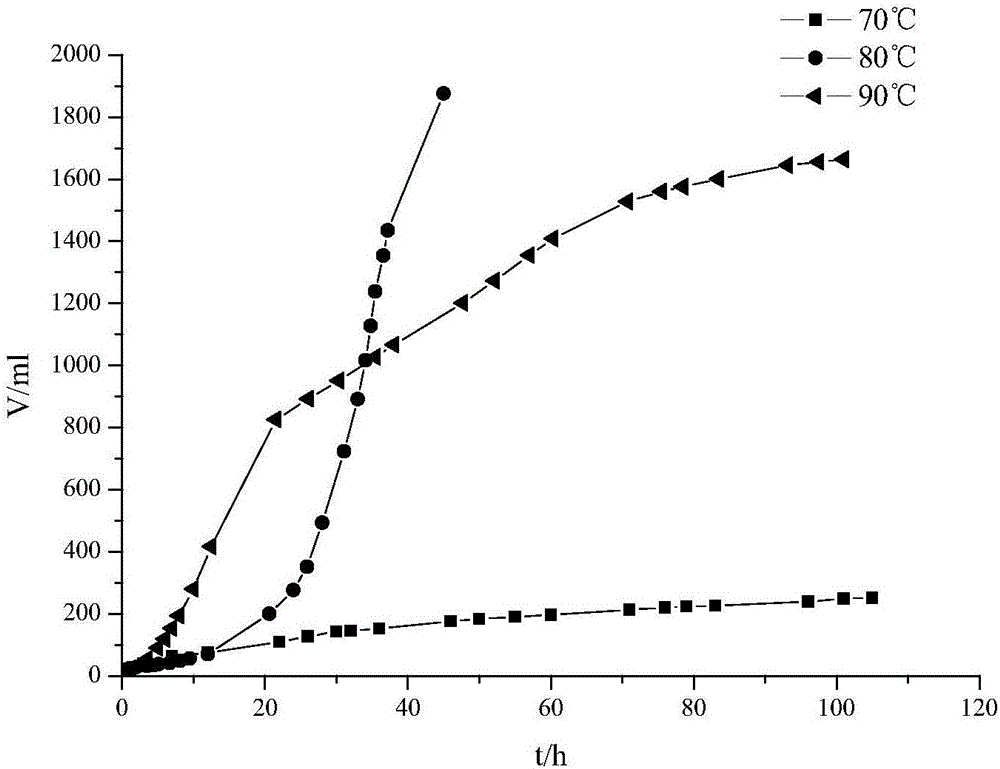
[0035] (2) HCO 2 H / NEt 3 System preparation: measure 3mL NEt 3 , 2mL anhydrous HCO 2 H. Prepare HCO with 2mL DMF 2 H / NEt 3 solution; under nitrogen protection, the HCO 2 H / NEt 3 - The DMF solution is frozen in liquid nitrogen until i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com