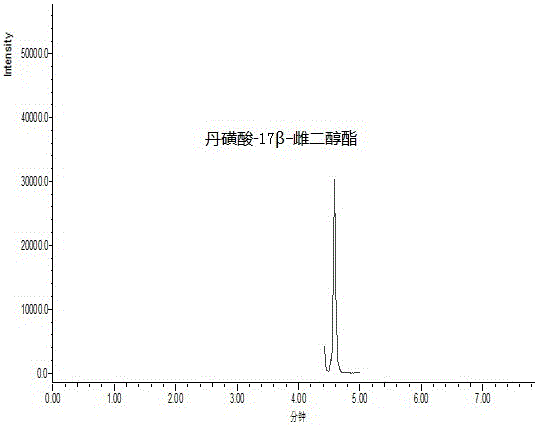
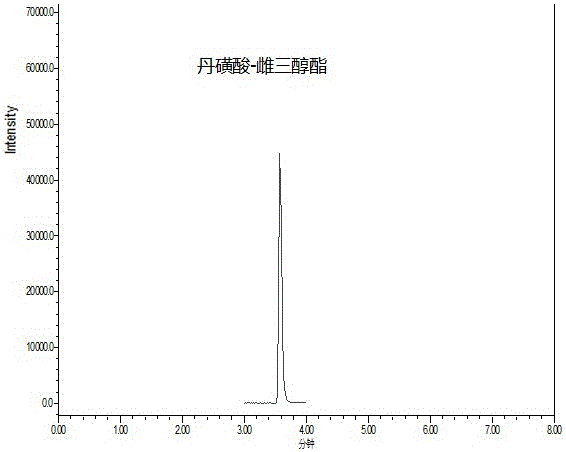
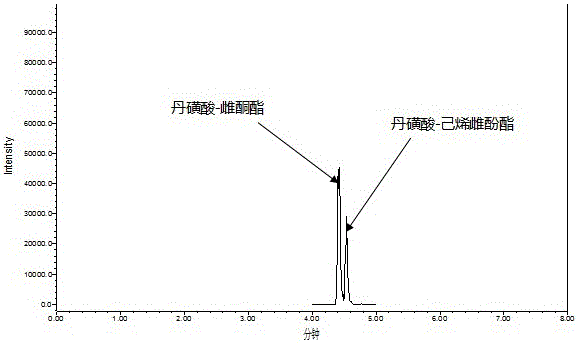
Method for measuring phenolic estrogen by means of derivation
A phenolic estrogen and derivatization technology, which is applied in the field of veterinary drug residue analysis and detection, can solve the problems of low sensitivity, low ionization efficiency, and short start-up vacuum time, etc., and achieves improved sensitivity, low detection cost, and good reproducibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1 Derivatization reaction conditions
[0054] ① Preparation of dansyl chloride derivatization reagent: Dissolve solid dansyl chloride in acetone solvent to prepare a dansyl chloride derivatization reagent with a concentration of 1 mg / mL, put it into a brown bottle, and store it in a -20°C refrigerator.
[0055] ② Derivatization reaction condition 1: Add 10 μL of 1 mg / mL dansyl chloride derivatization reagent prepared in step (1) ①, adjust the pH of the mixture to 8.0 with sodium bicarbonate, vortex mix for 30 seconds, and derivatize at 45°C for 10 minutes .
[0056] ③ Derivatization reaction condition 2: Add 100 μL of 1 mg / mL dansyl chloride derivatization reagent prepared in step (1) ①, adjust the pH of the mixture to 9.0 with sodium bicarbonate, vortex mix for 30 seconds, and derivatize at a temperature of 55°C for 10 minutes .
[0057] ④ Derivatization reaction condition 3: Add 200 μL of the 1 mg / mL dansyl chloride derivatization reagent prepared in step...
Embodiment 2
[0060] The preparation of embodiment 2 estrogen standard samples and estrogen standard curve drawing
[0061] (1) Preparation of estrogen standard samples
[0062] ①Preparation of standard stock solution: Dissolve the following six estrogen solid standards: 17β-estradiol, estriol, estrone, diethylstilbestrol, hexestrol, and diethylstilbestrol, respectively, in methanol to prepare The following 6 standard stock solutions with a concentration of 1mg / mL:
[0063] 1mg / mL 17β-estradiol standard stock solution;
[0064] 1mg / mL estriol standard stock solution;
[0065] 1mg / mL estrone standard stock solution;
[0066] 1mg / mL diethylstilbestrol standard stock solution;
[0067] 1mg / mL standard stock solution of hexestrol;
[0068] Dienestrol standard stock solution of 1mg / mL;
[0069] The above six standard stock solutions were stored in a -20°C refrigerator.
[0070] ②Preparation of five serial concentrations of mixed standard solutions: measuring step (1) ① The prepared concen...
Embodiment 3
[0095] Example 3 milk sample detection
[0096] (1) Extraction of milk samples
[0097] The market randomly selects milk as the experimental sample, and simultaneously detects the above six estrogen residues. A total of 3 added concentrations were set, which were 2, 10, and 50 μg / kg. A blank sample was set up, each concentration was repeated 6 times, and the repeatability experiment was carried out, and the recovery rate and variation coefficient were calculated respectively.
[0098] (2) Derivatization reaction
[0099] Accurately weigh 5 g of sample, add 100 μL of the internal standard with a concentration of 500 ng / mL prepared in step (1) ③ of Example 2, collect the eluate after extraction and purification, blow dry with nitrogen, add a volume of 200 μL in step (1) of Example 2 ) ④ prepare 1mg / mL dansyl chloride derivatization reagent, adjust the pH to 10 with sodium bicarbonate, vortex mix for 30s, and react at 60°C for 30min to obtain the derivatized product. Take it ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com