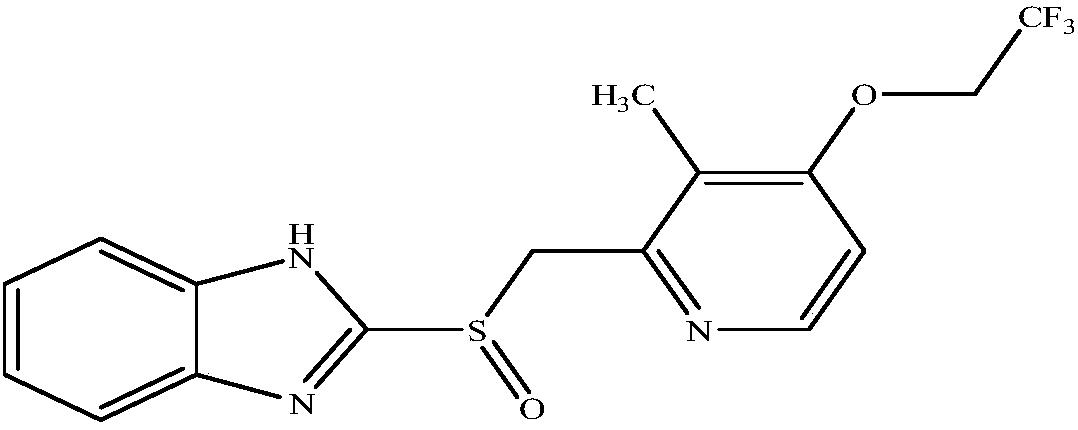
A kind of lansoprazole freeze-dried powder for injection and preparation method thereof
A technology of lansoprazole and lyophilized powder, applied in the field of biomedicine, can solve the problems of high risk of clinical application, threat to patient medication safety, calcium deficiency, etc., and achieves accelerated dissolution rate, water sublimation, and good redissolving performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] prescription
[0028] Element
Dosage
Lansoprazole
20g
40g
10g
Adjust the pH to 10.5
Water for Injection
Add to 3000ml
[0029] Preparation:
[0030] (1) Dosing: inject 2700ml water for injection into the dosing tank, cool to 30°C, add 40g hydroxyethyl starch, add 10g acetamide and stir to dissolve, add 20g lansoprazole, stir to make lansoprazole dissolve, Adjust the pH to 10.5 with sodium hydroxide, add water to 3000ml, add 0.1% (w / v) activated carbon to the liquid medicine, stir at room temperature for 20 minutes, filter for decarbonization, and filter the filtrate through a 0.22 μm filter membrane to sterilize;
[0031] (2) Filling and half stoppering;
[0032] (3) Freeze-drying: Pre-freezing adopts the method of repeated heating and cooling, which is characterized in that the temperature is reduced to -45 °C at a cooling rate of 1 °C / min, kept for 1 h...
Embodiment 2
[0035] prescription
[0036] Element
Dosage
Lansoprazole
30g
50g
20g
Adjust the pH to 11.0
Water for Injection
Add to 3000ml
[0037] Preparation:
[0038] (1) Dosing: inject 2700ml water for injection into the dosing tank, cool to 0°C, add 50g hydroxyethyl starch, add 20g acetamide and stir to dissolve, add 30g lansoprazole, stir to make lansoprazole dissolve, Adjust the pH to 11.0 with sodium hydroxide, add water to 3000ml, add 0.1% (w / v) activated carbon to the liquid medicine, stir at room temperature for 20 minutes, filter and decarbonize, and filter the filtrate through a 0.22 μm filter membrane to sterilize;
[0039] (2) Filling and half stoppering;
[0040] (3) Freeze-drying: Pre-freezing adopts the method of repeated heating and cooling, which is characterized in that the cooling rate is 1.5 °C / min down to -45 °C, kept for 2 hours, and then raised to -...
Embodiment 3
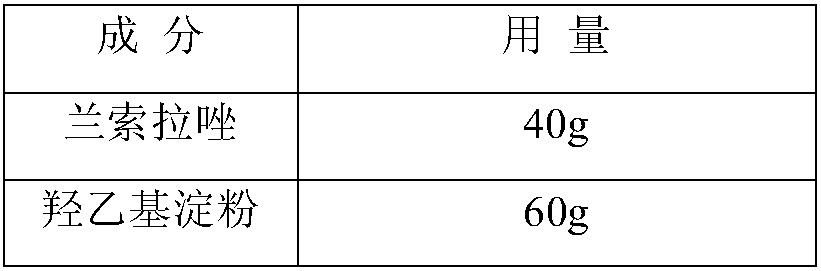
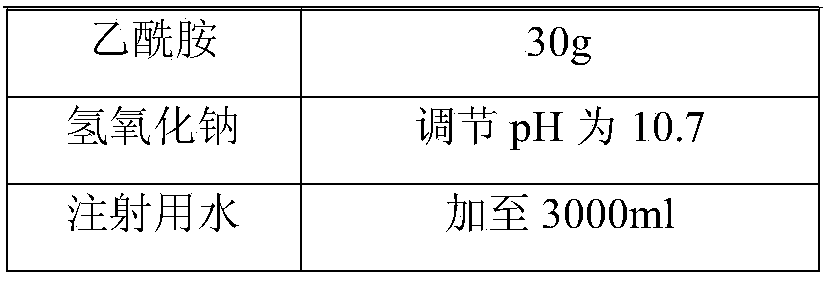
[0043] prescription
[0044]
[0045]
[0046] Preparation:
[0047](1) Dosing: inject 2700ml water for injection into the dosing tank, cool to 15°C, add 60g hydroxyethyl starch, add 30g acetamide and stir to dissolve, add 40g lansoprazole, stir to make lansoprazole dissolve, Use sodium hydroxide to adjust the pH to 10.7, add water to 3000ml, add 0.1% (w / v) activated carbon to the liquid medicine, stir at room temperature for 20 minutes, filter for decarbonization, and filter the filtrate through a 0.22 μm membrane filter to sterilize;
[0048] (2) Filling and half stoppering;
[0049] (3) Freeze-drying: Pre-freezing adopts the method of repeated temperature rise and fall pre-freeze, which is characterized in that the temperature rise and fall rate is reduced to -45°C at a cooling rate of 1.2°C / min, kept for 1.5h, and then raised at a heating rate of 2°C / min. Heat at -25°C for 1.5h, then cool down to -40°C at a cooling rate of 1.2°C / min, and hold for 2h; sublimate and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com