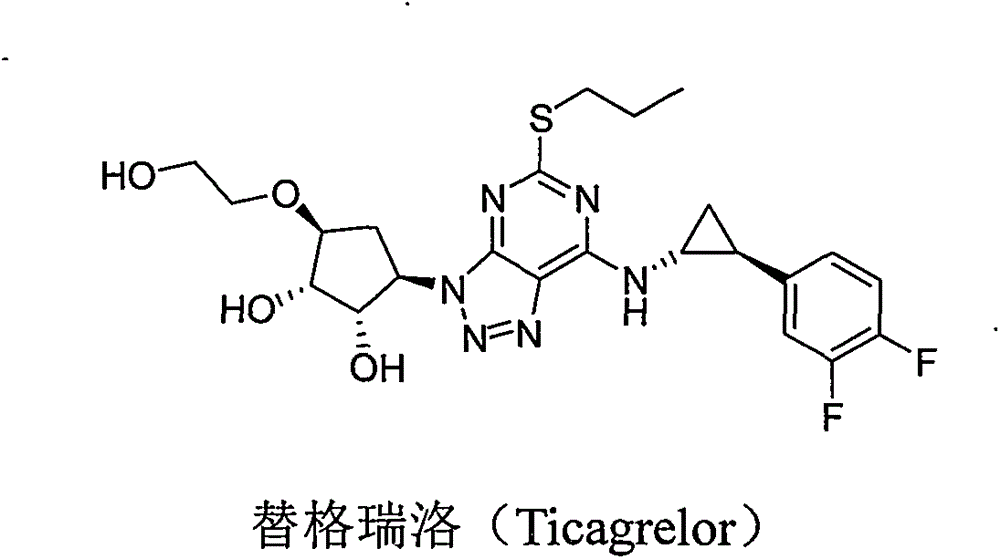
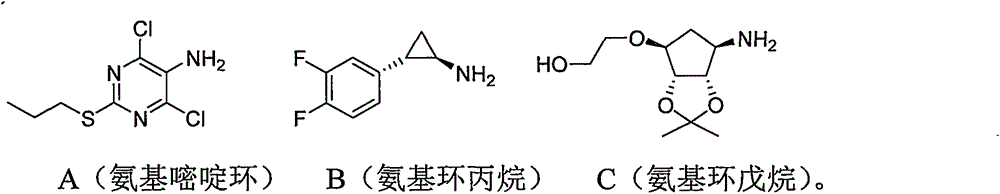
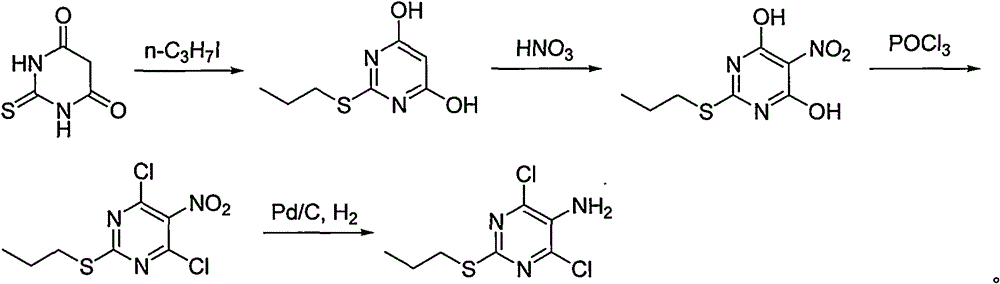
Preparing method for ticagrelor intermediate 4,6-dichloro-2-tri-sulfydryl-5-aminopyrimidine
A technology of propylmercaptopyrimidine and ticagrelor, which is applied in the direction of organic chemistry, can solve the problems of instability, low yield, and increase the difficulty of impurity separation, so as to control the generation of impurities, reduce the possibility of impurity generation, and avoid The effect of catalytic hydrogenation on the risk of dechlorination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add 10.0kg (75.69mol) of dimethyl malonate into a 50L glass reactor, start stirring, control the temperature in an ice-water bath to about 10°C, and slowly add 9.54kg of fuming nitric acid dropwise. After the dropwise addition was completed, the reaction was continued at a temperature of 5-10°C for 6 hours. Add 20L of saline solution, release the solution and move it into a 100L reaction kettle, add 20L of ethyl acetate, stir for 10 minutes, and let stand to separate layers. The ethyl acetate layer was washed twice with 10% sodium carbonate 20, and then washed with distilled water until the pH was 7. The ethyl acetate layer was dried with anhydrous sodium sulfate, and the ethyl acetate was evaporated to collect fractions at 98-102°C (1mmHg). 10.26 kg of dimethyl 2-nitromalonate was obtained with a molar yield of 76.5%.
Embodiment 2
[0039] Add methanol 30L and thiourea 6.45kg (84.69mol) into the 100L reactor, start stirring and dissolving, add 10.0kg (56.46mol) of dimethyl 2-nitromalonate, raise the temperature to reflux, slowly add 30% methanol dropwise Sodium methanol solution 15kg, react for 10 hours after the dropwise addition, TLC shows that the reaction is complete, stop stirring, cool to 50°C, release the reaction solution, continue to cool to room temperature, precipitate a white solid, add 6N hydrochloric acid to adjust the pH to 7 , centrifuged and filtered to obtain a white solid, which was washed several times with water and vacuum-dried to obtain 9.22 kg of 5-nitro-2-thiobarbituric acid with a molar yield of 86.4%.
Embodiment 3
[0041]Add 50L of methanol and 50kg of 10% NaOH solution into a 200L reactor, start stirring, add 9.0kg (47.58mol) of 5-nitro-2-thiobarbituric acid, and dropwise add 9.70kg (57.09mol) of 1-iodopropane , the dropwise addition was completed, and the reaction was carried out at room temperature for 10 hours, and TLC showed that the reaction was complete. Release the reaction solution, adjust the pH to 2-3 with 6N hydrochloric acid, and precipitate a large amount of solids, centrifugally filter and wash with distilled water to obtain a white solid, which is dried in vacuum to obtain 4,6-dihydroxy-5-nitro-2-propylmercaptopyrimidine 9.71kg, molar yield 88.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com