Ligand for aggregation-induced emission and complexes
A technology of aggregation-induced luminescence and complexes, which is applied in the direction of luminescent materials, compounds of group 5/15 elements of the periodic table, preparations for in vivo tests, etc., and can solve problems such as overlap and interference of fluorescence emission spectra, and inability to obtain information
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
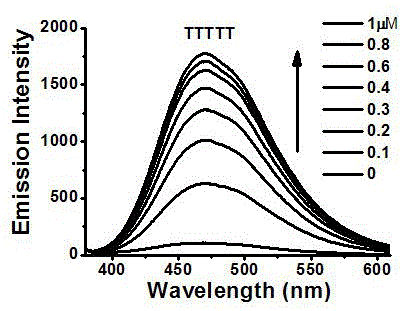
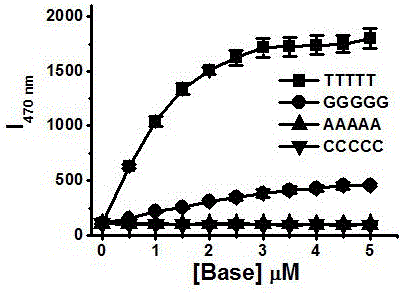
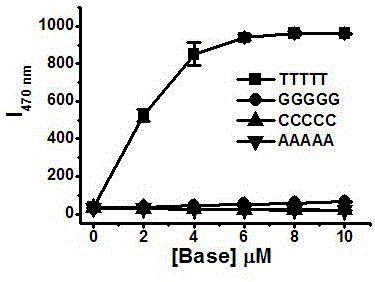
[0059] Embodiment 1, zinc, cadmium complex is used for the detection of nucleic acid
[0060] Synthesis of complex Zn1:
[0061]
[0062] Synthesis of Ligand 5: Put the raw material A1 (0.46g, 1mmol) and fenneline (0.69g, 4mmol) into a single-necked round-bottomed flask, add 40mL of anhydrous acetonitrile, reflux for 1 day, and distill under reduced pressure. After separation by column chromatography, 0.39 g of the target product was obtained with a yield of 71%. ESI-MS:m / z[M+H] + :547.Anal.Calcd for C 36 h 42 N 4 O: C, 79.08; H, 7.74; N, 10.25; Found: C, 78.94; H, 7.58; N, 10.07.
[0063] Preparation of complex Zn1: Dissolve 107mg (0.2mmol) of ligand 5 in 0.5mL methanol solution, and weigh Zn(NO 3 ) 2 ·6H 2 O 84mg (0.3mmoL) was dissolved in 0.5mL of methanol solution. The methanol solution of zinc nitrate was slowly added dropwise to the methanol solution of Ligand 5, during which a white solid was produced and precipitated, left for a period of time, filtered wit...
Embodiment 2
[0075] Embodiment 2, preparation and time-resolved detection of rare earth complexes
[0076] The synthetic route of complex Eu1 is as follows:
[0077]
[0078] Preparation of Ligand 8: Ligand 5 (0.08 g, 0.146 mmol), 2-chloro-N,N-diethylacetamide (0.11 g, 0.74 mmol), potassium iodide (0.20 g, 1.2 mmol) were added to a round bottom flask ), potassium carbonate (0.22g, 1.6mmol) and 10mL of acetonitrile, reflux reaction under argon protection for 48h, concentrated under reduced pressure, using chloroform-methanol (v:v=20:1) as eluent, passed through a silica gel column Purified by chromatography to obtain 0.12 g of a colorless oily liquid with a yield of 93%. ESI-MS:m / z[M+H] + :886,[M+Na] + :908.
[0079] Preparation of complex Eu1: Add ligand 8 (0.048g, 0.053mmol), hexahydrate, europium trichloride (0.022g, 0.06mmol) and 2.5mL methanol to a one-necked bottle, and reflux for 15h, then collect the filtrate by filtration. Distilled under reduced pressure to obtain a yellow...
Embodiment 3
[0086] Embodiment 3, the synthesis of magnetic contrast agent
[0087] The synthetic routes of ligand 9 and ligand 10 are as follows:
[0088]
[0089] Synthesis of Ligand 9: Ligand 5 (0.11g, 0.2mmol), tert-butyl bromoacetate (0.4g, 4mmol) and 10mL of acetonitrile were added to a round-bottomed flask. (v:v=20:1) was used as the eluent, and purified by silica gel column chromatography to obtain 0.15 g of a colorless oily liquid with a yield of 84%. ESI-MS:m / z[M+H] + :889.
[0090] Synthesis of Ligand 10: Ligand 9 (0.09 g, 0.1 mmol) and 5 mL of concentrated hydrochloric acid were added to a round bottom flask, stirred for 4 h, then concentrated under reduced pressure at 70°C to obtain about 0.06 g of white solid product with a yield of 83%. ESI-MS:m / z[M-H] - :719.
[0091] By implementing the method in Example 2, the ligand 10 was mixed with EuCl 3 , GdCl 3 , MnCl 2 reaction, the corresponding complex can be obtained, the structure is as follows:
[0092]
[0093]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com