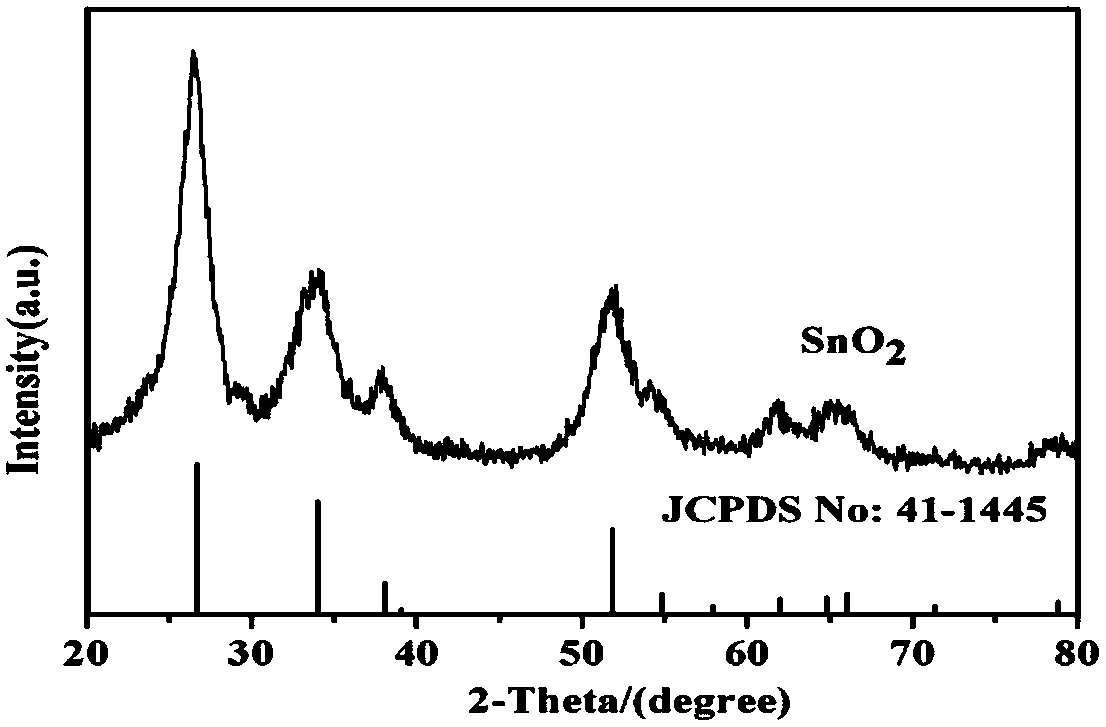
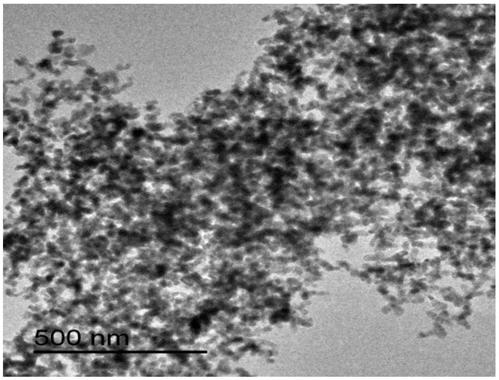
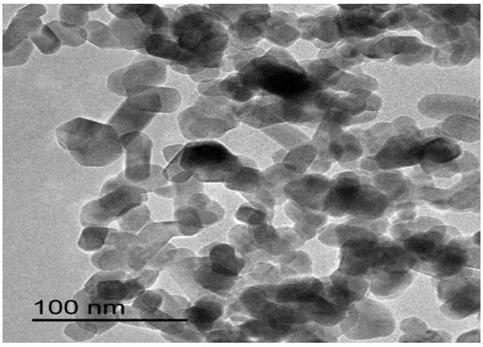
A kind of sno2 nanorod for negative electrode of lithium ion battery and preparation method thereof
A lithium-ion battery and nanorod technology, applied in battery electrodes, nanotechnology for materials and surface science, nanotechnology, etc., can solve the problems of cyclohexane toxic substance reaction cycle, limited wide application, high production cost, etc. Achieve the effects of improving cycle stability and specific capacity, increasing transmission rate, and shortening cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Add 0.451g SnCl 2 ·2H 2 Add O into 40mL deionized water, stir for 5min to SnCl 2 ·2H 2 After the O is completely dissolved, a milky white solution is obtained. Add 0.05g of super P with a particle size of 30-40 nm to the milky white solution, and then ultrasonically treat it for 20 minutes at a power of 80W and a temperature of 30°C to make super P and Sn 2+ Full effect to get a uniform mixture, Sn in the mixture 2+ The concentration is 0.05mol·L -1 .
[0027] (2) Transfer the mixed liquid to the hydrothermal reactor, the filling degree of the reactor is 60%, and put the hydrothermal reactor into the MDS-10 high-throughput ultra-high pressure closed microwave digestion instrument; select the temperature control mode for reaction, The temperature control mode is: the reaction temperature is 120° C., the reaction time is 10 minutes, and the reaction is naturally cooled to room temperature after the reaction is completed.
[0028] (3) Centrifuge and wash the product obtained...
Embodiment 2
[0031] (1) Put 0.846g SnCl 2 ·2H 2 Add O into 50mL deionized water, stir for 5min to SnCl 2 ·2H 2 After the O is completely dissolved, a milky white solution is obtained. Add 0.1g of super P with a particle size of 30-40 nm to the milky white solution, and then sonicate it for 20 minutes at a power of 80W and a temperature of 50°C to make super P and Sn 2+ Full effect to get a uniform mixture, Sn in the mixture 2+ The concentration is 0.075mol·L -1 .
[0032] (2) Transfer the mixed liquid to the hydrothermal reactor, the filling degree of the reactor is 80%, and put the hydrothermal reactor into the MDS-10 high-throughput ultra-high pressure closed microwave digestion apparatus; select the temperature control mode for reaction, The temperature control mode is: the reaction temperature is 120°C, the reaction time is 20min, and the reaction is naturally cooled to room temperature after the reaction.
[0033] (3) Centrifuge and wash the product obtained in step (2) at a rotation speed ...
Embodiment 3
[0037] (1) Add 1.128g SnCl 2 ·2H 2 Add O into 80mL deionized water, stir for 5min to SnCl 2 ·2H 2 After O is completely dissolved, a milky white solution is obtained. Add 0.2g of super P with a particle size of 30-40nm to the milky white solution, and then sonicate it for 20 minutes at a power of 100W and a temperature of 50℃ to make super P and Sn 2+ Full effect to get a uniform mixture, Sn in the mixture 2+ The concentration is 0.062mol·L -1 .
[0038] (2) Transfer the mixed liquid to the hydrothermal reactor, the filling degree of the reactor is 70%, and put the hydrothermal reactor into the MDS-10 high-flux ultra-high pressure closed microwave digestion instrument; select the temperature control mode for reaction, The temperature control mode is: the reaction temperature is 150° C., the reaction time is 20 min, and the reaction is naturally cooled to room temperature after the reaction.
[0039] (3) Centrifuge and wash the product obtained in step (2) at a rotation speed of 9000...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com