A targeted nano drug-carrying system for auxiliary immune cell therapy and its preparation method
A technology of immune cell therapy and immune cells, which is applied in the field of targeted nano-drug delivery system, can solve the problems of high dosage of anti-tumor drugs for tumor cells, inability to fully identify tumor cells, and large toxic and side effects of normal cells, so as to avoid steric space Resistant effect, avoid toxic side effects, low toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
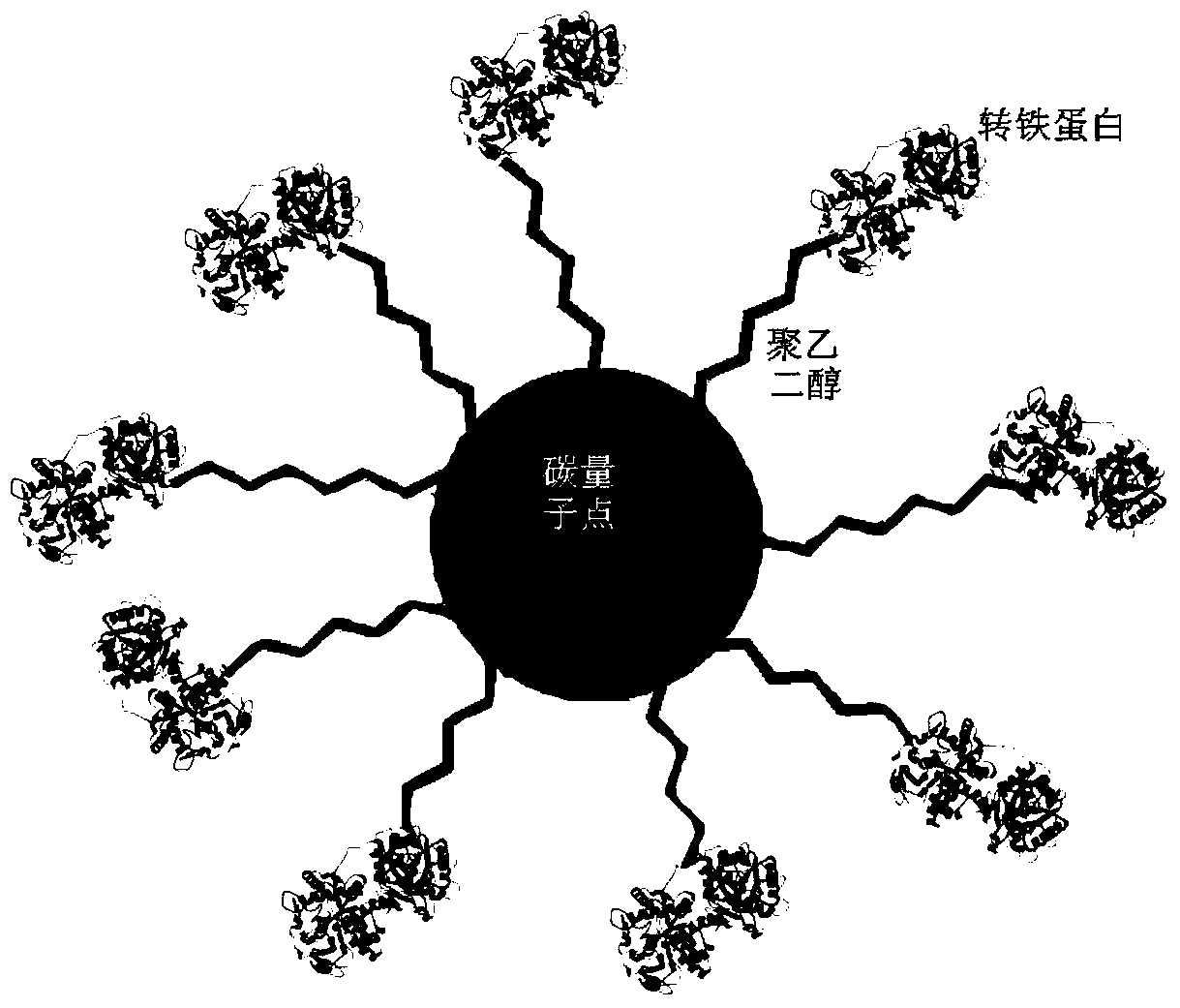
[0048] The targeted nano-drug loading system of the present invention uses nano-material carbon quantum dots CQDs as the carrier, polyethylene glycol PEG as the cross-linked product, transferrin Tf as the targeting molecule, and is formed by covalent coupling. The nano-carrier CQDs-PEG-Tf has a particle size of 224.5±90.2nm. structure as figure 1 shown.
[0049] Its preparation process is as follows:
[0050] (1) Preparation of carrier carbon quantum dots-polyethylene glycol (CQDs—PEG—OH)
[0051] Dissolve 0.2g of ascorbic acid in 2mL of ultrapure water, and stir it magnetically for 15min to form a transparent and uniform solution. The solvent filter is filtered, and washed and purified in a dialysis bag (molecular weight cut off: 8000) to obtain an aqueous solution of carbon quantum dots. The obtained aqueous solution of carbon quantum dots is evaporated in a rotary evaporator to remove most of the solvent, and then dried in a vacuum oven at 35-65° C. to obtain carbon qua...
Embodiment 2
[0065] The targeted nano-drug loading system of the present invention uses nano-material carbon quantum dots CQDs as the carrier, polyethylene glycol PEG as the cross-linked product, transferrin Tf as the targeting molecule, and is formed by covalent coupling. The nano-carrier CQDs-PEG-Tf has a particle size of 224.5±90.2nm. structured as figure 1 shown.
[0066] Its preparation process is as follows:
[0067] (1) Preparation of carrier carbon quantum dots-polyethylene glycol (CQDs—PEG—OH)
[0068] Dissolve 0.2g of ascorbic acid in 2mL of ultrapure water, stir it magnetically for 15min to form a transparent and uniform solution, add 4mL of PEG-200 and stir for 20min, use a microwave power of 300W, set the temperature at 85°C, and microwave for 140s. The solvent filter is filtered, and washed and purified in a dialysis bag (molecular weight cut off: 8000) to obtain an aqueous solution of carbon quantum dots. The obtained aqueous solution of carbon quantum dots is evaporated...
Embodiment 3
[0082] The targeted nano-drug loading system of the present invention uses nano-material carbon quantum dots CQDs as the carrier, polyethylene glycol PEG as the cross-linked product, transferrin Tf as the targeting molecule, and is formed by covalent coupling. The nano-carrier CQDs-PEG-Tf has a particle size of 224.5±90.2nm. structured as figure 1 shown.
[0083] Its preparation process is as follows:
[0084] (1) Preparation of carrier carbon quantum dots-polyethylene glycol (CQDs—PEG—OH)
[0085] Dissolve 0.2g of ascorbic acid in 2mL of ultrapure water, stir it magnetically for 15min to form a transparent and uniform solution, add 4mL of PEG-200 and stir for 20min, use a microwave power of 400W, set the temperature at 100°C, and microwave for 200s. The solvent filter is filtered, and washed and purified in a dialysis bag (molecular weight cut off: 8000) to obtain an aqueous solution of carbon quantum dots. The obtained aqueous solution of carbon quantum dots is evaporate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com