Drug eluting stent and manufacturing method and application thereof
A technology for eluting stents and drugs, applied in the field of drug-eluting stents and its preparation, can solve problems such as adverse effects, inconvenient treatment, and patient inconvenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
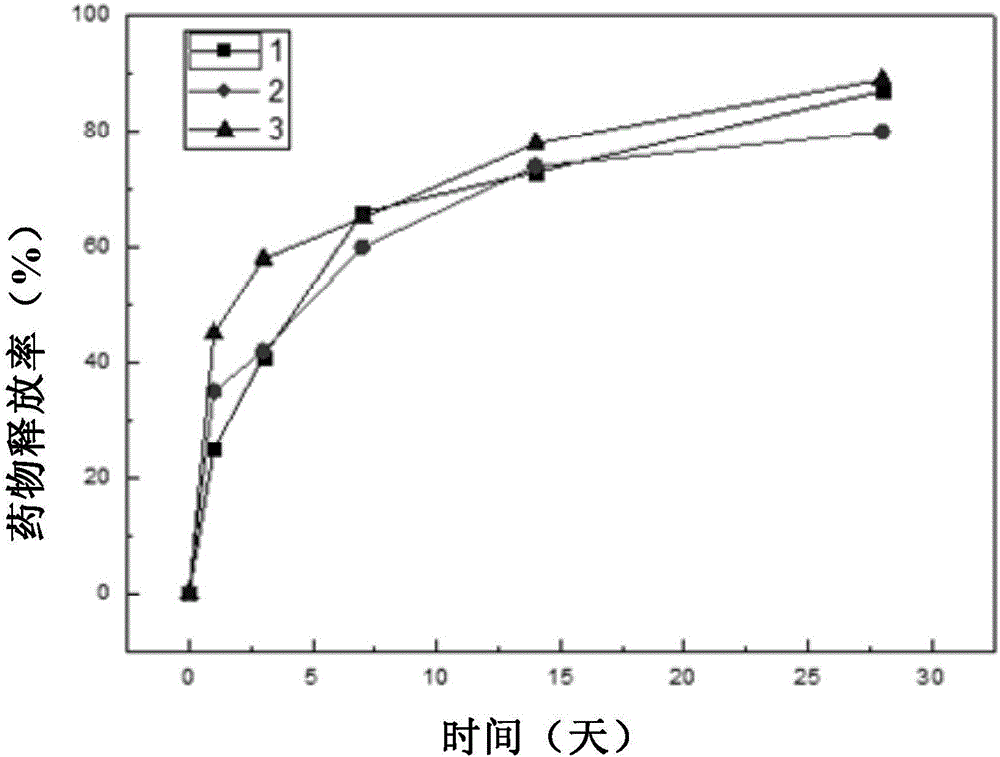
Examples
Embodiment 1
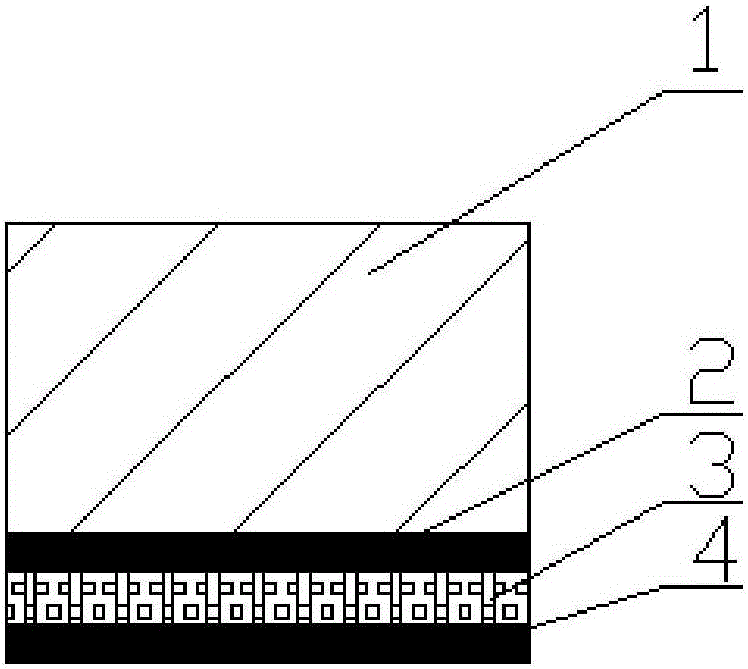
[0049] Dissolve 0.5 g of PLGA with a number average molecular weight of about 200,000 Daltons in 12.5 mL of tetrahydrofuran solvent, and mix and stir to form a uniform solution. Utilize ultrasonic atomization equipment to spray the solution evenly on the inner surface of the prepared stent base (1), and dry it with an inert gas (argon) at 60° C. for 24 hours to form a drug-coated degradable polymer layer ( 2).
[0050] Dissolve the total mass of 0.1g rapamycin and antiplatelet drug (aspirin and clopidogrel mixed drug) in 2.5mL tetrahydrofuran solvent at a mass ratio of 5:1, stir thoroughly to make active drug solution, and put it in the stent Atomized spraying is carried out on the first layer of coating, and the number of spraying is controlled to control the drug loading at about 200μg / cm 2 , and then dried with an inert gas at 60° C. for 24 hours to form a drug layer (3) of drug coating.
[0051] Then spray PLGA and tetrahydrofuran solvent evenly on the drug layer (3) to ...
Embodiment 2
[0053] Dissolve 0.5 g of PLGA with a molecular weight of about 400,000 Daltons in 12.5 mL of tetrahydrofuran solvent, and mix and stir to form a uniform solution. Utilize ultrasonic atomization equipment to spray the solution evenly on the inner surface of the prepared stent base (1), and dry it with an inert gas (argon) at 70° C. for 24 hours to form a drug-coated degradable polymer layer ( 2).
[0054] The total mass of 0.1g rapamycin and antiplatelet drug (aspirin and clopidogrel mixture) was dissolved in 2.5mL tetrahydrofuran solvent with a mass ratio of 1:1, and the active drug solution was made after fully stirring. Atomized spraying is carried out on the inner coating of the stent, and the number of sprayings is controlled to control the drug loading at about 200 μg / cm 2 , and then dried with an inert gas at 60° C. for 24 hours to form a drug layer (3) of drug coating.
[0055] Then uniformly spray PLGA and tetrahydrofuran solvent on the drug layer (3) and stir thorou...
Embodiment 3
[0057] Dissolve 0.5 g of PLGA with a molecular weight of about 200,000 Daltons in 12.5 mL of tetrahydrofuran solvent, and mix and stir to form a uniform solution. Utilize ultrasonic atomization equipment to spray the solution evenly on the inner surface of the prepared stent base (1), and dry it with an inert gas (argon) at 40° C. for 48 hours to form a drug-coated degradable polymer layer ( 2).
[0058] The total mass is 0.1g rapamycin and antiplatelet drug (mixture of aspirin and clopidogrel) with a mass ratio of 5:1 dissolved in 2.5mL tetrahydrofuran solvent, fully stirred to make active drug solution, in Atomized spraying is carried out on the inner coating of the stent, and the number of sprayings is controlled to control the drug loading at about 500 μg / cm 2 , and then dried with an inert gas at 70° C. for 12 hours to form a drug layer (3) of drug coating.
[0059] Then uniformly spray PLGA and tetrahydrofuran solvent on the drug layer (3) and stir thoroughly to make a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap