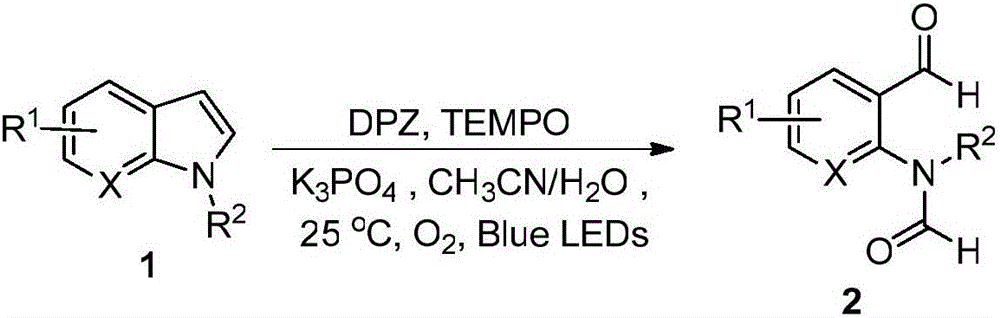
Method for preparing N-(2-formyl phenyl) N-substituted formamide derivatives by means of visible light catalysis
A technology for the preparation of formylphenyl and catalysis, which is applied in chemical instruments and methods, the preparation of organic compounds, and the preparation of carboxylic acid amides. It can solve the problems of poor selectivity, failure to obtain the target product, and complexity, and achieve low cost. , simple post-processing, high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
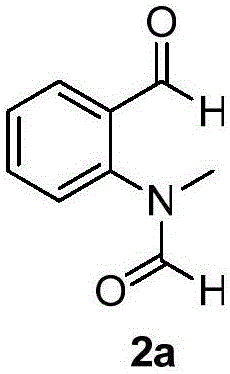
[0021] The structural formula of compound 2a is as follows:
[0022]
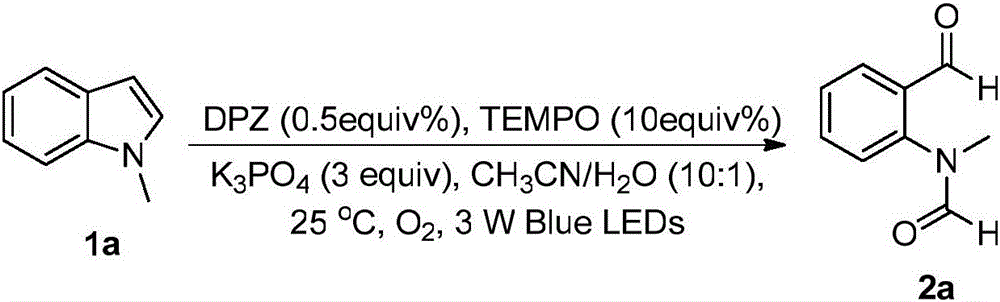
[0023] The synthetic route of compound 2a is as follows:
[0024]
[0025] The synthetic steps of compound 2a are as follows:
[0026] (1) Add 28 μL (0.0005 mmol, 0.005 equiv) of DPZ solution (1 mg dissolved in 160 μl acetonitrile) into a 10 mL reaction flask, and then remove the solvent by rotary evaporation;
[0027] (2) Then add magneton, N-methylindole 1a (0.1mmol, 1.0equiv), solvent (2.0mL, CH 3 CN:H 2 O volume ratio=10:1), K 3 PO 4 (0.3mmol, 3equiv.) and 10equiv% TEMPO (0.01mmol) as additives, seal the reaction bottle, and insert an oxygen bulb on the bottle stopper to provide oxygen to the reaction bottle;
[0028] (3) Put the reaction bottle into a 25°C incubator, and stir the reaction at a distance of 5cm from a 3W blue light (λ=450-455nm); (4) The reaction is detected by a TLC plate. After the reaction is complete, extract with ethyl acetate , spin-dried, passed through the column (col...
Embodiment 2
[0031]
[0032] Product Name: N-(2-Formylphenyl)-N-Benzylformamide
[0033] In step (2), N-methylindole 1a was replaced with p-N-benzylindole 1b, and other preparation steps and purification methods were carried out with reference to Example 1; yellow oil, 96% yield. 1 H NMR (300MHz, CDCl 3 )δ9.77 (s, 1H), 8.64 and 8.27 (s, 1H, CHO rotameric), 7.92–7.87 (m, 1H), 7.64–7.55 (m, 1H), 7.43–7.51 (m, J=11.3, 7.5,1H), 7.31–7.28(m,1H), 7.25–7.03(m,5H), 4.98 and 4.83(s,2H,CH 2 rotameric). 13 C NMR (75MHz, CDCl 3 )δ189.2, 189.0, 162.8, 162.3, 142.0, 139.9, 135.3, 135.2, 134.9, 132.8, 132.3, 130.4, 129.2, 129.1, 129.0, 128.8, 128.6, 128.6, 128.5, 128.2 HR, 58.2 m / z 240.1032(M+H + ), calc.for C 15 h 14 NO 2 240.1025.
Embodiment 3
[0035]
[0036] Product name: N-(2-formylphenyl)-N-phenylcarboxamide
[0037] In step (2), p-N-phenylindole 1 was used to replace N-methylindole 1a, and other preparation steps and purification methods were carried out with reference to Example 1; yellow oil, 85% yield. 1HNMR (300MHz, CDCl3) δ10.14 and 10.07 (s, 1H, CHOrotameric), 8.84 and 8.54 (s, 1H, CHO rotameric), 8.01 (dd, J = 7.7, 1.6, 1H), 7.73-7.65 (m, 1H),7.60–7.48(m,1H),7.43–7.27(m,4H),7.25–7.13(m,2H).13C NMR(75MHz,CDCl3)δ189.0,188.9,162.3,161.6,141.6,140.4,135.4 ,135.1,132.2,130.6,130.2,129.9,129.7,129.3,128.9,128.7,126.9,126.6,124.5,123.4.HRMS(ESI)m / z 226.0873(M+H + ), calc.for C 14 h 12 NO 2 226.0868.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com