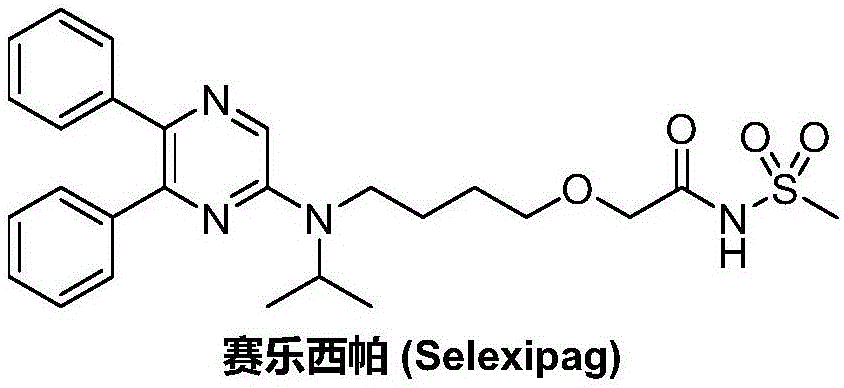
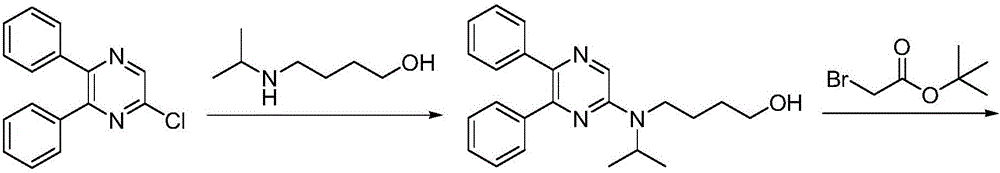
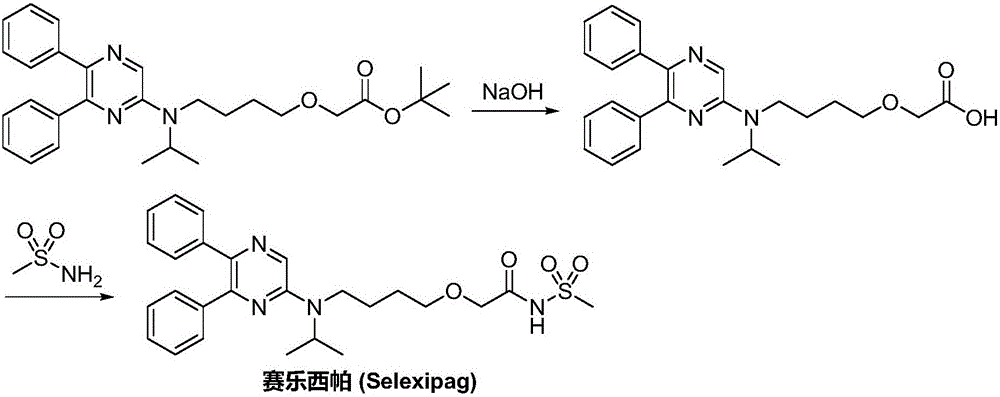
Synthetic method of selexipag
A synthetic method, the technology of Selexipa, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of complex and cumbersome operation, short process flow, unfavorable scale-up production and industrial promotion, etc., and achieve reasonable technical scheme, high yield and simplified operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A) Preparation of [4-(tert-butoxycarbonyl)(isopropyl)aminobutoxy]acetate tert-butyl ester:
[0028] 4-[(tert-butoxycarbonyl)(isopropyl)amino]-1-butanol (20.0 g, 0.09 mol) and tert-butyl bromoacetate (21.1 g, 0.11 mol) were dissolved in dichloromethane (90 mL), Tetrabutylammonium chloride (0.72g, 2.6mmol), potassium hydroxide (7.3g, 0.13mol) and water (12.0g) were added, the reaction mixture was stirred and reacted at 25°C for 2 hours, and the reaction solution was concentrated to dryness by rotary evaporation under reduced pressure. , added ethyl acetate for extraction, dried over magnesium sulfate, concentrated to dryness by rotary evaporation, and recrystallized from a mixed solvent of ethanol and isopropanol to obtain [4-(tert-butoxycarbonyl)(isopropyl)aminobutoxy]acetate tert-butyl Ester, pale yellow oil (26.6g), yield 89.0%, the reaction formula of this step is as follows:
[0029]
[0030] B) Preparation of [4-(tert-butoxycarbonyl)(isopropyl)aminobutoxy]acetic...
Embodiment 2
[0039] A) Preparation of [4-(tert-butoxycarbonyl)(isopropyl)aminobutoxy]acetate tert-butyl ester:
[0040] 4-[(tert-butoxycarbonyl)(isopropyl)amino]-1-butanol (23.0g, 0.10mol) and tert-butyl bromoacetate (25.2g, 0.13mol) were dissolved in 1,2-dichloroethyl Alkane (110mL), tetrabutylammonium bromide (1.1g, 3.5mmol), sodium hydroxide (6.4g, 0.16mol) and water (14.0g) were added, the reaction mixture was stirred at 30°C for 3 hours, and the reaction solution was depressurized Concentrate by rotary evaporation to dryness, add ethyl acetate for extraction, dry over magnesium sulfate, concentrate by rotary evaporation to dryness, recrystallize from a mixed solvent of ethanol and isopropanol to obtain [4-(tert-butoxycarbonyl)(isopropyl)aminobutoxy Base] tert-butyl acetate, light yellow oil (30.3g), yield 88.2%, the reaction formula of this step is the same as embodiment 1;
[0041] B) Preparation of [4-(tert-butoxycarbonyl)(isopropyl)aminobutoxy]acetic acid:
[0042] [4-(tert-butox...
Embodiment 3
[0047] A) Preparation of [4-(tert-butoxycarbonyl)(isopropyl)aminobutoxy]acetate tert-butyl ester:
[0048] 4-[(tert-butoxycarbonyl)(isopropyl)amino]-1-butanol (12g, 0.05mol) and tert-butyl bromoacetate (12.1g, 0.06mol) were dissolved in chloroform (70mL), and tetrabutyl Ammonium iodide (0.5g, 1.3mmol), lithium hydroxide (1.7g, 0.07mol) and water (6.5g), the reaction mixture was stirred at 20°C for 4 hours, the reaction solution was concentrated to dryness by rotary evaporation under reduced pressure, and acetic acid was added Extracted with ethyl ester, dried over magnesium sulfate, concentrated to dryness by rotary evaporation, recrystallized from a mixed solvent of ethanol and isopropanol to obtain [4-(tert-butoxycarbonyl)(isopropyl)aminobutoxy]acetate tert-butyl ester, shallow Yellow oil (15.6g), yield 86.8%, the reaction formula of this step is the same as Example 1;
[0049] B) Preparation of [4-(tert-butoxycarbonyl)(isopropyl)aminobutoxy]acetic acid:
[0050] [4-(tert-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com