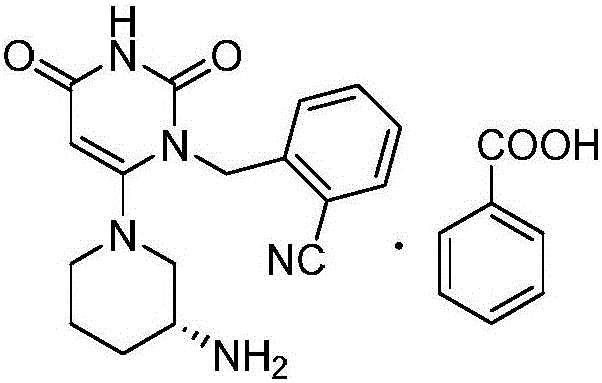
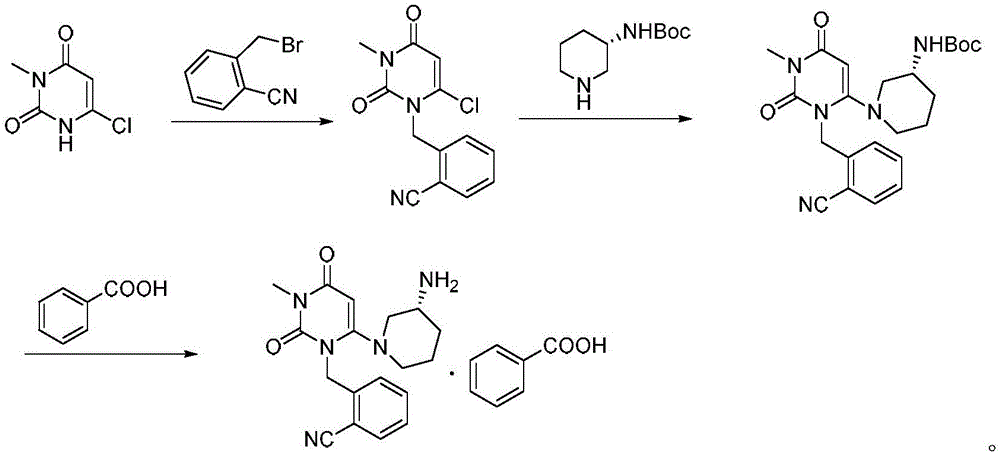
Method for preparing drug Alogliptin for treating diabetes type II
A technology for a diabetes drug and methyluracil, applied in the field of drug synthesis, can solve the problems of cumbersome steps, long reaction time, low product yield and the like, and achieve the effects of simple operation steps, shortened reaction time, and avoidance of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A kind of method for preparing treatment type II diabetes medicine alogliptin, the method comprises the following steps:
[0030] 1) Under nitrogen protection, copper acetate 9.1g (50mmol), triethylamine 25.3g (250mmol), potassium iodide 6.6g (40mmol), 6-chloro-3-methyluracil 16.1g (100mmol) and 2-cyano 22.5g (115mmol) of benzyl bromide was carried out contact reaction in 200ml of acetonitrile at 45°C for 1.5 hours. After the reaction, the reaction solution was poured into water, filtered, washed with water, and dried to obtain 6-chloro-1-(2-isocyanobenzyl 26.5 g of )-3-methylpyrimidine-2,4-(1H,3H)-dione, the yield was 96.2%, and the purity was 98.79%.
[0031] 2) 13.8 g (50 mmol) of 6-chloro-1-(2-isocyanobenzyl)-3-methylpyrimidine-2,4-(1H,3H)-dione, (R)-3-tert-butyl 12.0g (60mmol) of oxycarbonylaminopiperidine and 15.2g (110mmol) of potassium carbonate were mixed in DMF at 65°C for 3 hours. After the reaction, water was added, extracted with dichloromethane, concentra...
Embodiment 2
[0034] A kind of method for preparing treatment type II diabetes medicine alogliptin, the method comprises the following steps:
[0035] 1) Under nitrogen protection, 9.1g (50mmol) of copper acetate, 25.3g (250mmol) of triethylamine, 5.0g (30mmol) of potassium iodide, 16.1g (100mmol) of 6-chloro-3-methyluracil and 2-cyano 23.5g (120mmol) of benzyl bromide was carried out contact reaction in 200ml of acetonitrile at 40°C for 2 hours. After the reaction, the reaction solution was poured into water, filtered, washed with water, and dried to obtain 6-chloro-1-(2-isocyanobenzyl 26.2 g of )-3-methylpyrimidine-2,4-(1H,3H)-dione, the yield was 95.1%, and the purity was 98.64%.
[0036] 2) 13.8 g (50 mmol) of 6-chloro-1-(2-isocyanobenzyl)-3-methylpyrimidine-2,4-(1H,3H)-dione, (R)-3-tert-butyl 11.0g (55mmol) of oxycarbonylaminopiperidine and 20.7g (150mmol) of potassium carbonate were mixed in DMF at 70°C for 3 hours. After the reaction, water was added, extracted with dichloromethane,...
Embodiment 3
[0039] A kind of method for preparing treatment type II diabetes medicine alogliptin, the method comprises the following steps:
[0040] 1) Under nitrogen protection, 10.9g (60mmol) of copper acetate, 20.2g (200mmol) of triethylamine, 8.3g (50mmol) of potassium iodide, 16.1g (100mmol) of 6-chloro-3-methyluracil and 2-cyano 21.6g (110mmol) of benzyl bromide was subjected to a contact reaction at 50°C in 200ml of acetonitrile for 2 hours. After the reaction, the reaction solution was poured into water, filtered, washed with water, and dried to obtain 6-chloro-1-(2-isocyanobenzyl 26.2 g of )-3-methylpyrimidine-2,4-(1H,3H)-dione, the yield was 95.1%, and the purity was 97.73%.
[0041] 2) 13.8 g (50 mmol) of 6-chloro-1-(2-isocyanobenzyl)-3-methylpyrimidine-2,4-(1H,3H)-dione, (R)-3-tert-butyl 11.0 g (55 mmol) of oxycarbonylaminopiperidine and 17.3 g (125 mmol) of potassium carbonate were mixed in DMF at 65°C for 2.5 hours. After the reaction, water was added, extracted with dichlo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com