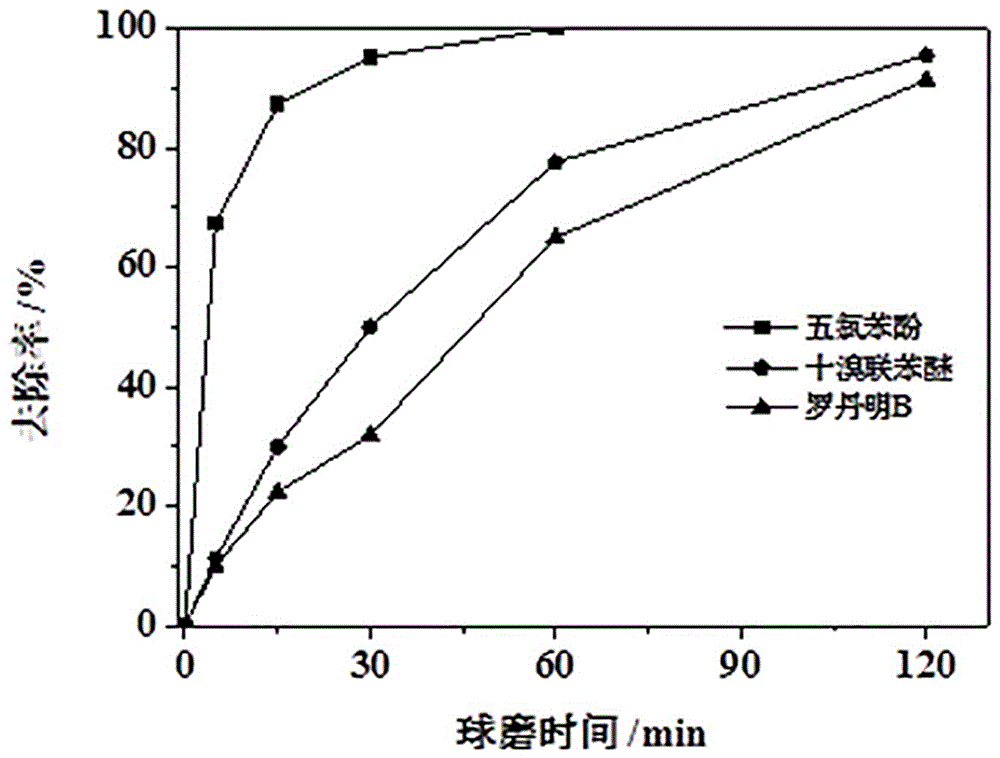
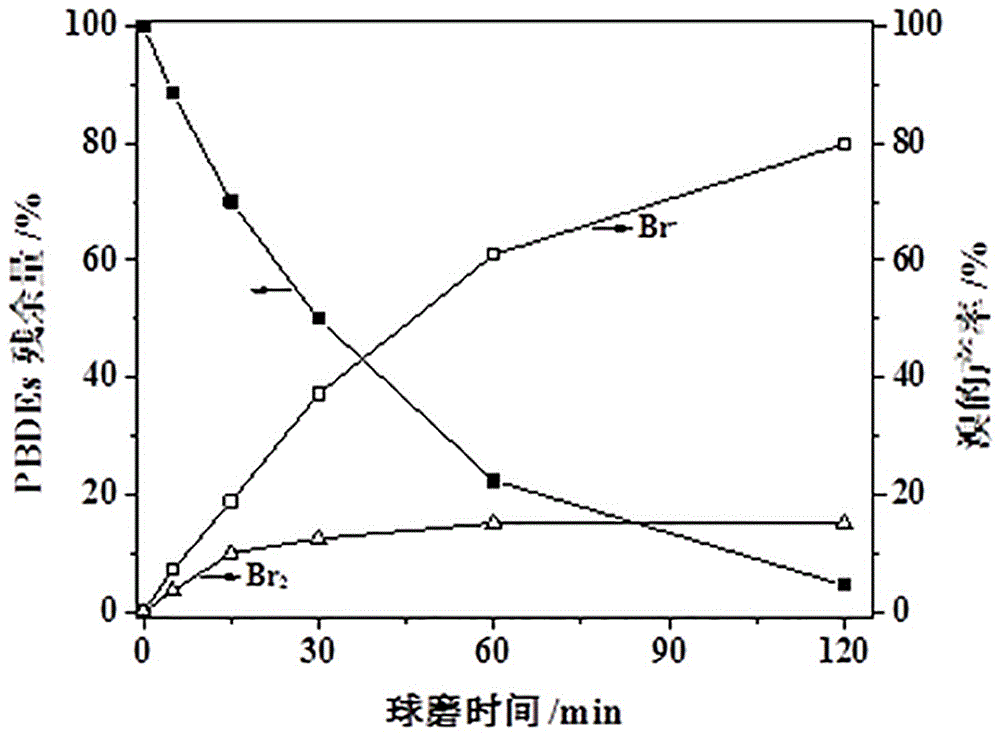
Method for treating organic solid waste through manganese dioxide, preparing manganic oxide and treating water pollutants through manganic oxide
A technology of manganese trioxide and manganese dioxide, which is applied in the field of treatment and the preparation of new materials, can solve the problems of destroying the composition structure, secondary pollution, toxic large dioxins, etc., and achieves high activity, low cost, and complete realization. Effects of degradation and mineralization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Step 1: Mix manganese dioxide and decabromodiphenyl ether solid powder in a mortar according to the material molar ratio of 100:1, and then transfer to a dry stainless steel ball mill jar. Then add 50 stainless steel grinding balls into the tank, the diameter of the grinding balls is 10 mm, and the weight is 4 g. The volume of the ball mill jar is 250 mL, the inner depth of the jar body is 70 mm, and the inner diameter of the jar mouth is 77 mm. The ball mill jar and the ball mill cover are connected by a sealing ring.
[0033] Step 2: Fix the ball mill jar on the ball mill, set the rotation speed of the ball mill jar to 350 rpm, conduct the ball mill reaction at normal temperature and pressure, and change the revolution direction of the ball mill once every 15 minutes of reaction. After the ball milling reaction was carried out for 5, 15, 30, 60 and 120 min respectively, the ball milling tank was taken out, and the gas and solid powder in the tank were collected.
[...
Embodiment 2
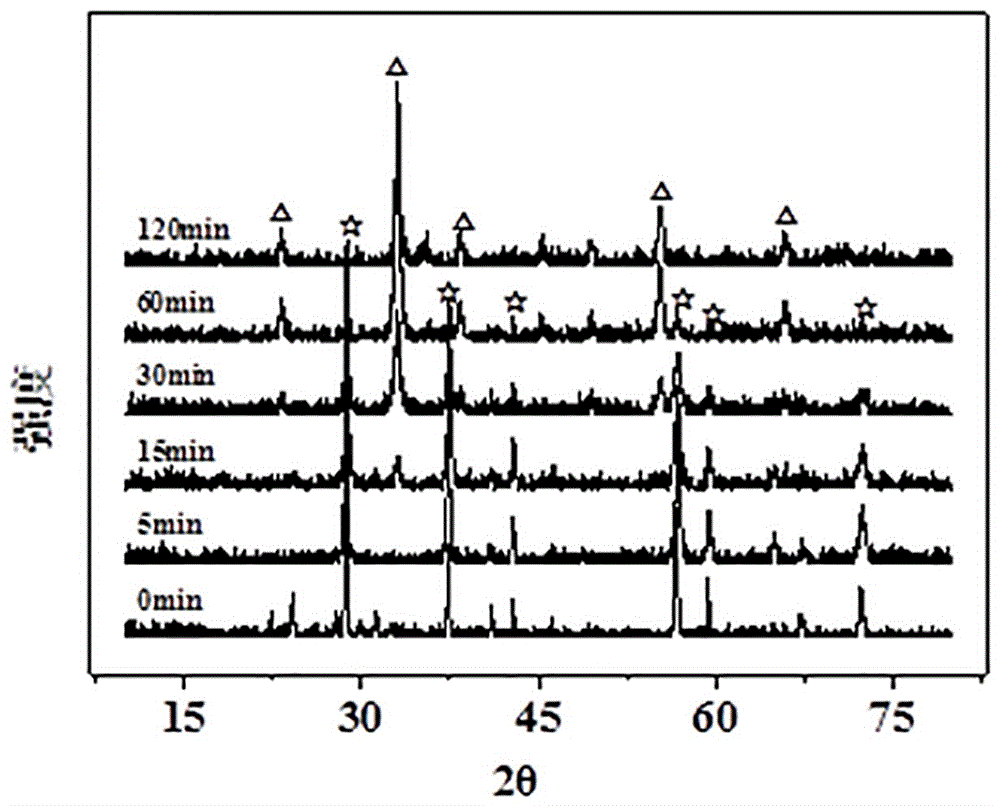
[0038] In order to monitor the change of the material during the ball milling reaction, steps 1 and 2 in Example 1 were repeated, and samples were taken at different time points (0, 5, 15, 30, 60 and 120 min) of the ball milling reaction, and the samples were obtained The solid samples were subjected to X-ray diffraction (XRD) measurement, the obtained results are as follows image 3 shown. By comparison with the XRD standard card, it can be seen that the material before the reaction is mainly β-MnO 2 (Such as image 3 shown by ☆ in ); with the prolongation of ball milling reaction time, the β-MnO 2 The characteristic diffraction peaks gradually decrease, and at the same time, the 2θ is 23 o 、33 o and 55 o Nearby, some new diffraction peaks (such as image 3 Indicated by △). After comparative analysis, the new peak belongs to Mn 2 o 3 , indicating that some β-MnO 2 Converted to Mn by ball milling reaction 2 o 3 . When the ball milling reaction was carried out for ...
Embodiment 3
[0040] In order to evaluate the catalytic performance of the material after the ball milling reaction, the material after the reaction in Step 2 of Example 1 was collected. This material of 0.04g is added into the 250mL beaker that is filled with 100mL concentration and is 25mg / L phenol waste liquid, is placed on the magnetic stirrer, at 25 o After stirring and mixing at C for a certain period of time, 1 mL of 30 g / L persulfate was added thereto to start the catalytic degradation reaction of phenol. When the reaction was carried out for 2, 5, 10, 20, 30 and 60 min respectively, 750 μL of the reaction solution was taken out and placed in a 1.5 mL centrifuge tube, mixed with 750 μL of anhydrous methanol and centrifuged (14000 rpm) for 5 min to obtain the supernatant After the liquid was filtered through a 0.22 μm filter membrane, the residual phenol concentration in the filtrate was determined by high performance liquid chromatography, and the results were as follows: Figure 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com