Albendazole artificial antigen and preparation method and application thereof
A technology of albendazole and antigen, which is applied in the field of albendazole artificial antigen and its preparation, can solve the problems of cumbersome operation steps, inapplicability to large-scale sample screening and detection, and high cost, and achieve high purity, high yield, The effect of simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, the preparation of albendazole hapten
[0041] One, the preparation of albendazole hapten
[0042] Add 500mg of albendazole and 20ml of dimethylformamide (DMF) (as the solvent for the reaction) into a 50ml round-bottomed flask, dissolve it, add 580mg of 4-aminobutyric acid, react at 80°C for 5h, TLC detects that the raw materials are processed after the reaction is completed , the reaction solution was added dropwise to 10ml of ice water, a white solid was precipitated, filtered, washed with water and dried to obtain 320mg of the product.
[0043] The reaction equation is as follows:
[0044]
[0045] 2. Structural identification of albendazole hapten
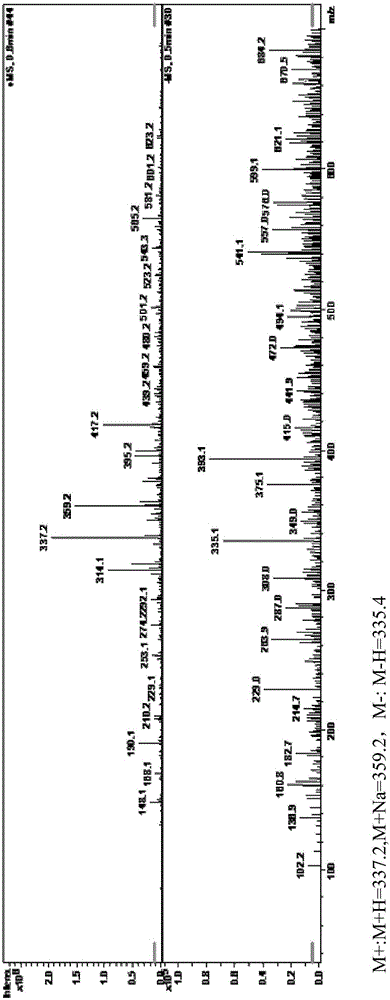
[0046] Mass spectrometry detection is carried out to gained 320mg product, M+:M+H=337.2, M+Na=359.2, M-:M-H=335.4, as figure 1 shown. The results show that its chemical structure is as shown in Formula I (MW=336.4), which is the albendazole hapten.
[0047]
Embodiment 2
[0048] Embodiment 2, preparation and structural identification of albendazole artificial antigen
[0049] 1. Preparation of albendazole artificial antigen
[0050] 1. Synthesis of immunogen
[0051] (1) Dissolve 15 mg of the hapten (Formula I) prepared in Example 1 in 1.5 mL of dimethylformamide (DMF). After dissolving completely, add 1-(3-dimethylaminopropyl)-3 - Ethylcarbodiimide hydrochloride (EDC) 26mg, N-hydroxysuccinimide (NHS) 25mg, room temperature (20-25°C) magnetic stirring reaction for 3h, to obtain solution I.
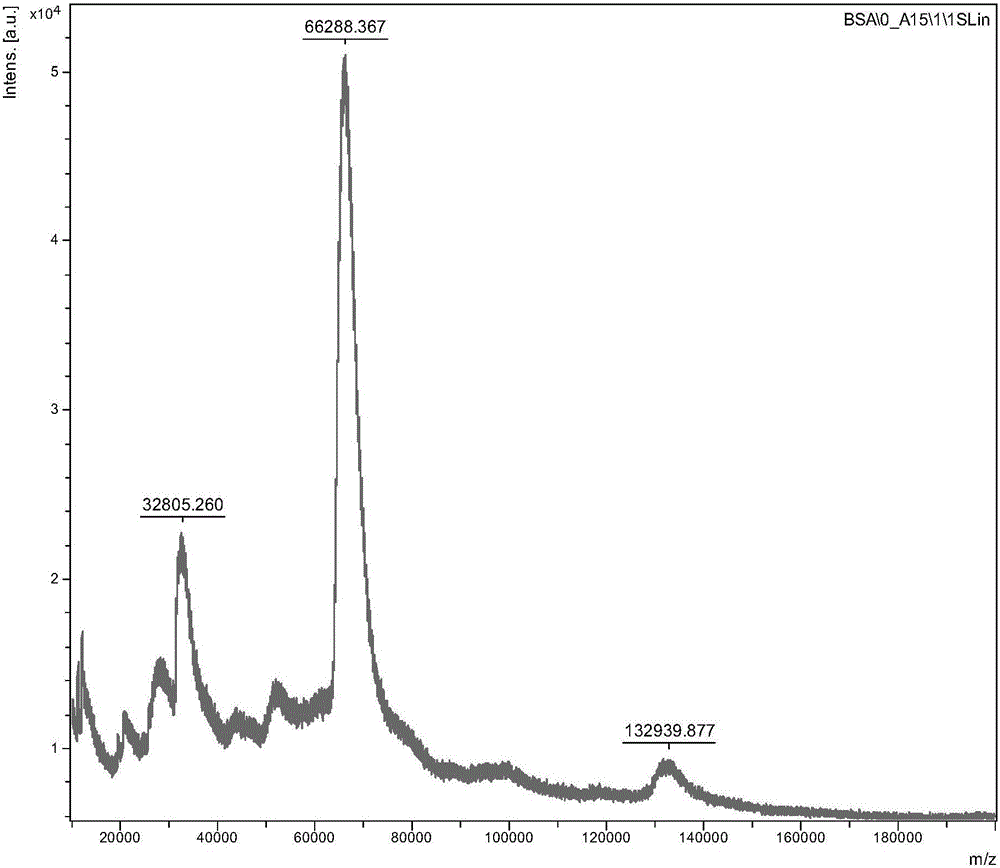
[0052](2) Weigh 50 mg of bovine serum albumin (BSA), dissolve it in 3.5 mL of 0.1 M carbonic acid buffer, stir at 400 rpm for 10 min, and fully dissolve to obtain solution II.
[0053] Wherein, the pH of the 0.1M carbonic acid buffer is 9.6, the solvent is water, and the solute and its concentration are as follows: Na 2 CO 3 1.59g / L, NaHCO 3 2.94g / L.
[0054] (3) Take the above solution I, add it dropwise to the above solution II in an ice-water bath...
Embodiment 3
[0064] Embodiment 3, albendazole artificial antigen immunization animal prepares antiserum
[0065] 1. Animal immunity
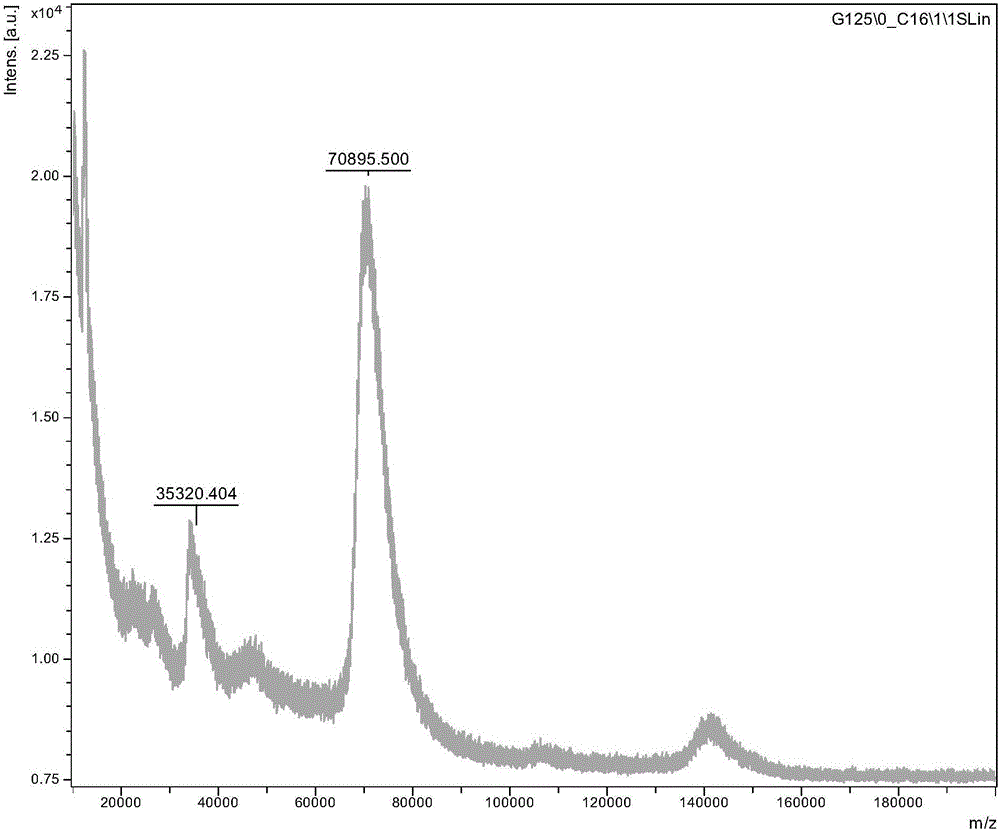
[0066] New Zealand white rabbits were immunized with the albendazole artificial antigen "albendazole-BSA" obtained in step Example 2 as an immunogen. The dose of each immunization is 100-200 μg, and the immunization method is multi-point subcutaneous injection on both shoulders and hind thighs, and about 1 / 4 of the immunogen is used in each area. For the first immunization, dilute the immunogen with normal saline, then mix it with Freund's incomplete adjuvant 1:1 (volume ratio) to make an emulsifier, and take the same dose of immunogen plus an equal volume of Freund's incomplete adjuvant every 2 weeks After mixing and emulsifying the vaccine, the immunization was boosted once. After 3 times of immunization in this way, the same dose of immunogen was added with Freund's incomplete adjuvant for the last immunization at an interval of 3 to 4 weeks. Blood was c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com