Oil-in-water nano-emulsion capable of obviously improving bioavailability of insoluble medicament and preparation method for oil-in-water nano-emulsion
A technology of insoluble drugs and oil-in-water type, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as increasing the bioavailability of insoluble drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
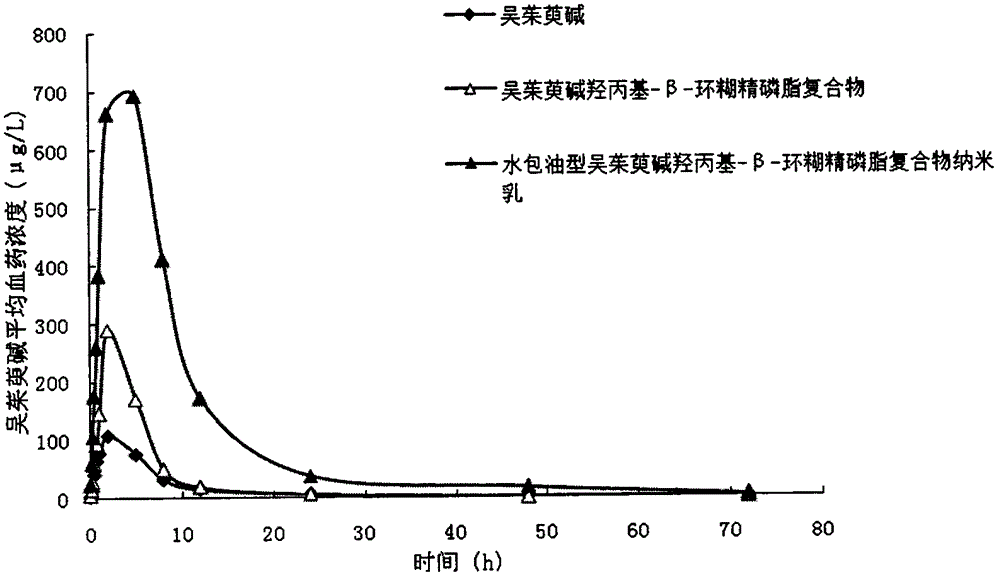
[0028] The weight ratio of each component contained in the insoluble drug cyclodextrin phospholipid complex formula is: 0.7 parts of evodiamine, 23.1 parts of soybean lecithin, 47.6 parts of α-cyclodextrin, and the oil-in-water nanoemulsion formula contains The weight ratio of each component is: 8.3 parts of evodiamine cyclodextrin phospholipid complex, 18.9 parts of ethyl oleate, 21.9 parts of polyoxyethylene (40) hydrogenated castor oil, 15.5 parts of ethanol, and 26.4 parts of distilled water.
[0029] The preparation method comprises the following steps: (1) Preparation of insoluble drug cyclodextrin phospholipid complex: take prescription amount of evodiamine, soybean lecithin, α-cyclodextrin, place in a round bottom flask, add ethanol, Magnetic stirring in a water bath at 40°C for 2 hours, and then rotary evaporation to remove the organic solvent to obtain the evodiamine cyclodextrin phospholipid complex; (2) Preparation of oil-in-water nanoemulsion: take the ethyl oleate...
Embodiment 2
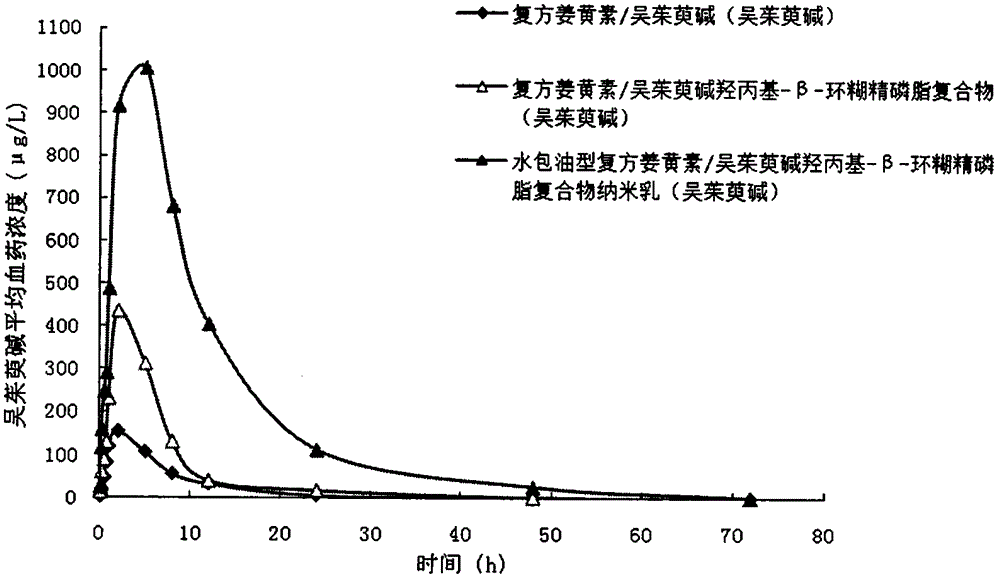
[0031] The weight ratio of each component contained in the insoluble drug cyclodextrin phospholipid complex formula is: 1.5 parts of curcumin and evodiamine (0.5 part of curcumin and 1 part of evodiamine), 27.7 parts of brain lecithin, β-cyclodextrin 30.4 parts of essence, the weight ratio of each component contained in the oil-in-water nanoemulsion formula is: compound curcumin / Evodia rutaecarpa reduced cyclodextrin phospholipid complex 10.2 parts, glyceryl monostearate 16.9 parts, monostearic acid 31.9 parts of propylene glycol, 17.2 parts of polyethylene glycol 400, and 28.5 parts of distilled water.
[0032] The preparation method includes the following steps: (1) Preparation of insoluble drug cyclodextrin phospholipid complex: take curcumin, evodiamine, brain lecithin, and β-cyclodextrin in the prescribed amount, place them in a round bottom flask, add In dichloromethane, stir magnetically in a water bath at 50°C for 3 hours and then remove the organic solvent by rotary e...
Embodiment 3
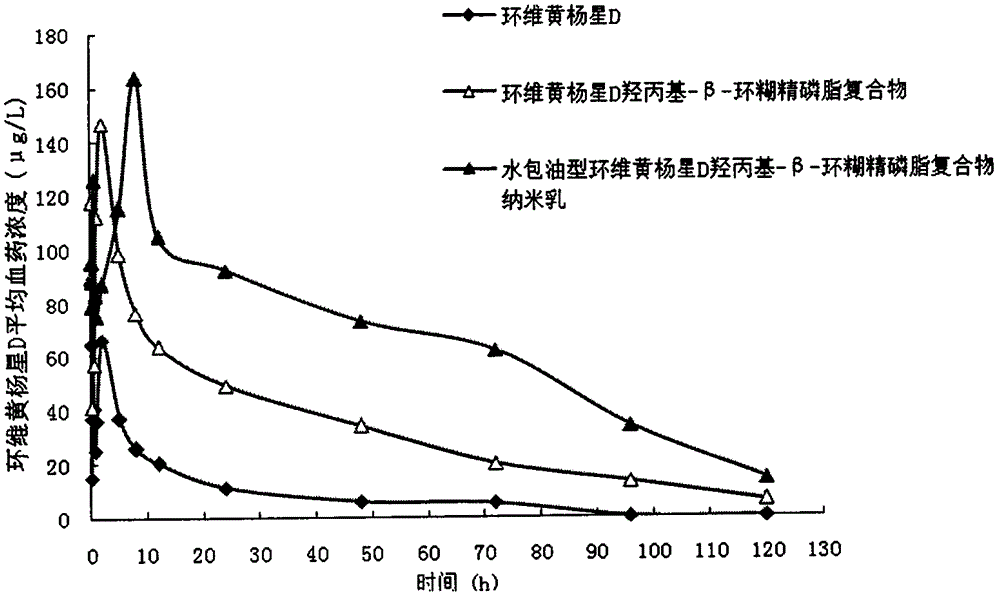
[0034] The weight ratio of each component contained in the insoluble drug cyclodextrin phospholipid complex formula is: cyclovir buxin D 1.2 parts, glycerophospholipid 3.4 parts, hydroxypropyl-α-cyclodextrin 6.4 parts, oil-in-water The weight ratio of each component contained in the type nanoemulsion formula is: 8.9 parts of Cyclovir buxus D cyclodextrin phospholipid complex, 11.9 parts of glyceryl monocaprylate, 25.7 parts of polyoxyethylene (40) hydrogenated castor oil, glycerin 25.7 parts of alcohol, 35.6 parts of distilled water.
[0035] The preparation method comprises the following steps: (1) Preparation of insoluble drug cyclodextrin phospholipid complex: take prescription amount of cyclovirbuxine D, glycerol lecithin, hydroxypropyl-alpha-cyclodextrin, and place in round bottom Add ethanol to the flask, stir magnetically in a water bath at 45°C for 4 hours, and then remove the organic solvent by rotary evaporation to obtain cyclovirbuxusin D cyclodextrin phospholipid c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com