Preparation method for synthesizing Fe3O4(PAA)@C-Au core-shell-structured microspheres with one-step hydrothermal method
A core-shell structure, hydrothermal technology, applied in catalyst activation/preparation, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve the problems of easy loss, easy agglomeration, etc. Easy to churn, short process, the effect of promoting the probability of collision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
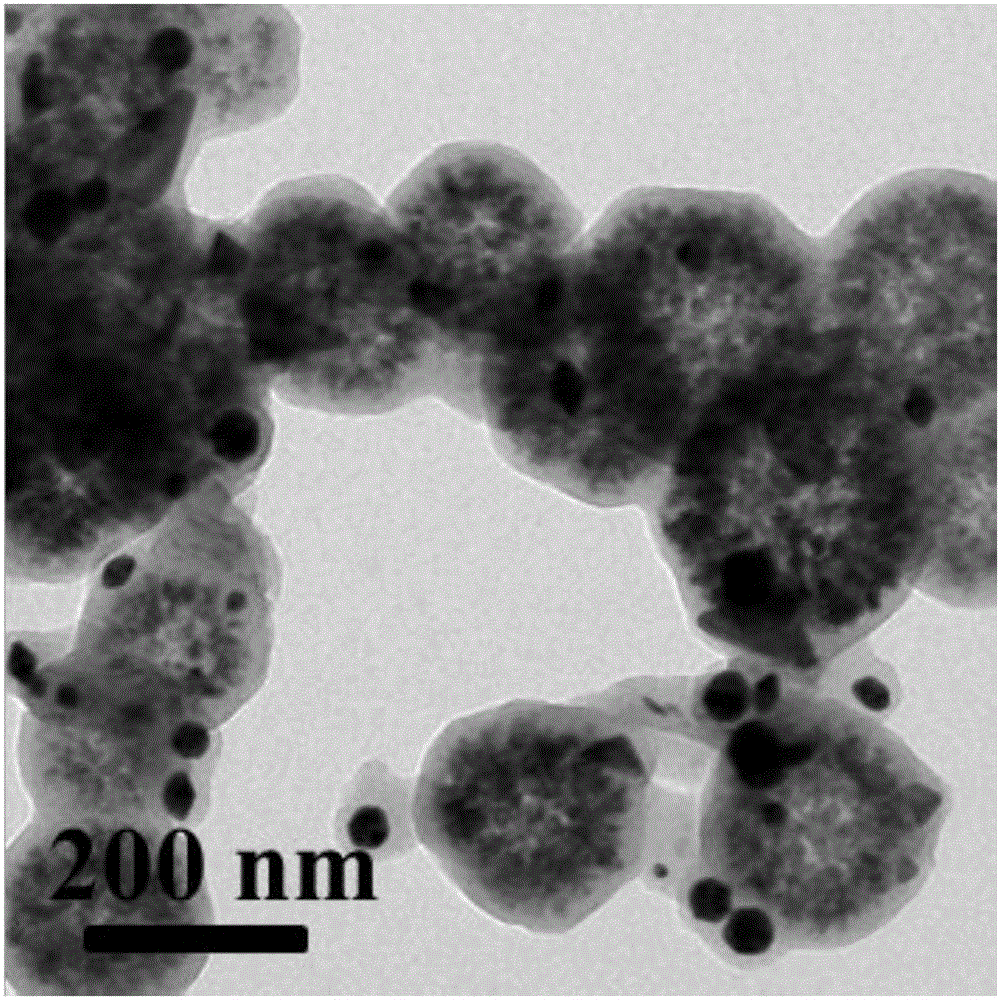
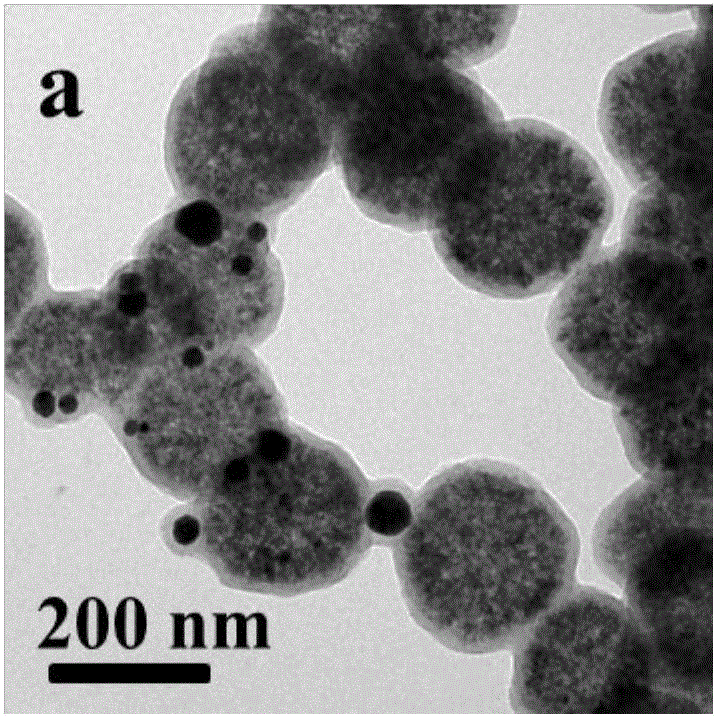
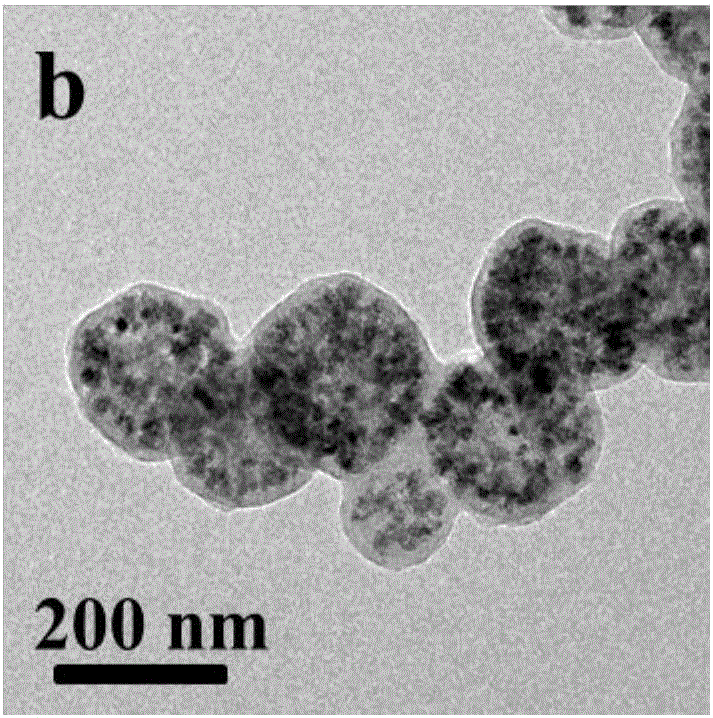
[0019] Under the condition of 60°C oil bath, disperse ferric chloride and polyacrylic acid in ethylene glycol, then add anhydrous sodium acetate and stir vigorously for 2 hours, after mixing evenly, transfer the obtained khaki precursor solution to an airtight container In the process, the temperature was controlled at 200° C. for 8 hours, and magnetic separation, alcohol washing, water washing, and drying were performed to obtain magnetic ferric oxide microspheres with a diameter of 260 nm. Among them, the mass ratio of ferric chloride, polyacrylic acid, ethylene glycol, and anhydrous sodium acetate is: 1:0.02:42:8;
[0020] Mix magnetic iron ferric oxide microspheres, glucose, deionized water, and chloroauric acid in a certain proportion by ultrasound, then transfer to a closed container, and control the temperature at 180°C for 6 hours to obtain a core-shell structure Fe 3 o 4 (PAA)@C-Au microspheres. Among them, the mass ratio of magnetic iron ferric oxide microspheres, ...
Embodiment example 2
[0022] Under the condition of 60°C oil bath, disperse ferric chloride and polyacrylic acid in ethylene glycol, then add anhydrous sodium acetate and stir vigorously for 2 hours, after mixing evenly, transfer the obtained khaki precursor solution to an airtight container In the process, the temperature was controlled at 200° C. for 8 hours, and magnetic separation, alcohol washing, water washing, and drying were performed to obtain magnetic ferric oxide microspheres with a diameter of 260 nm. Among them, the mass ratio of ferric chloride, polyacrylic acid, ethylene glycol, and anhydrous sodium acetate is: 1:0.02:42:8;
[0023] Mix magnetic iron ferric oxide microspheres, glucose, deionized water, chloroauric acid, and CTAB in a certain proportion, and then transfer them to an airtight container, and control the temperature at 180°C for 6 hours to obtain a core-shell structure Fe 3 o 4 (PAA)@C-Au microspheres. Among them, the mass ratio of magnetic iron ferric oxide microspher...
Embodiment example 3
[0025] Under the condition of 60°C oil bath, disperse ferric chloride and polyacrylic acid in ethylene glycol, then add anhydrous sodium acetate and stir vigorously for 2 hours, after mixing evenly, transfer the obtained khaki precursor solution to an airtight container In the process, the temperature was controlled at 200° C. for 8 hours, and magnetic separation, alcohol washing, water washing, and drying were performed to obtain magnetic ferric oxide microspheres with a diameter of 260 nm. Among them, the mass ratio of ferric chloride, polyacrylic acid, ethylene glycol, and anhydrous sodium acetate is: 1:0.02:42:8;
[0026] Mix iron ferric oxide microspheres, glucose, deionized water, chloroauric acid, and sodium citrate in a certain proportion by ultrasound, then transfer the magnetism to a closed container, and control the temperature at 180°C for 6 hours to obtain a core-shell structure Fe 3 o 4 (PAA)@C-Au microspheres. Among them, the mass ratio of magnetic iron ferri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com