Stent and medicine delivery device
A technology for delivering devices and drugs, which is applied to stents, devices for drugs, devices for human tubular structures, etc., can solve problems such as difficult to achieve, unsatisfactory effects, and difficult development, and achieve the effect of preventing the elastic retraction of blood vessels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
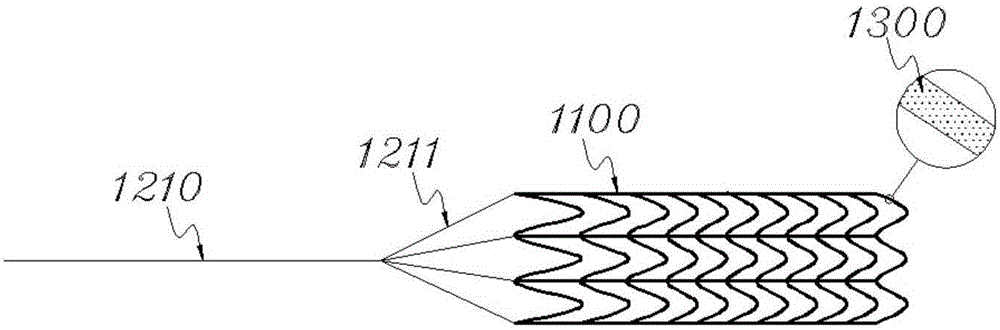
[0030] See figure 1 The stent 1100 provided in this embodiment includes a tubular stent body braided in a "W" shape by a plurality of nickel-titanium alloy wires, the weaving density of the tubular stent body gradually decreases from one end to the other end, wherein the loose ends form the connecting structure of the bracket 1100, from figure 1 It can be seen from the figure that the connection structure includes a plurality of connection terminals.
[0031] Dissolve 75mg of paclitaxel and 45mg of polyurea in 2-4ml of acetone and ethanol to form a mixed solution, spray the above mixed solution on the surface of the stent main body, and dry to obtain the drug coating 1300, thereby forming a drug-loaded stent.
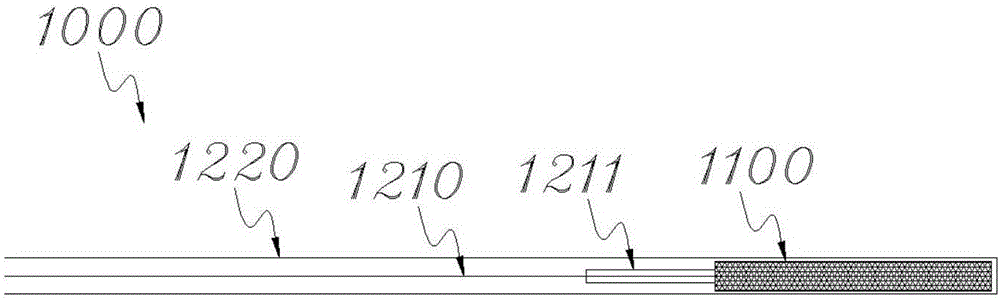
[0032] The stent 1100 of this embodiment is a recyclable stent, which can be used in conjunction with a recovery device during use. In this embodiment, the recovery device includes an introducer sheath 1220 (see figure 2 ) and a recycling wire 1210, one end of the...
Embodiment 2
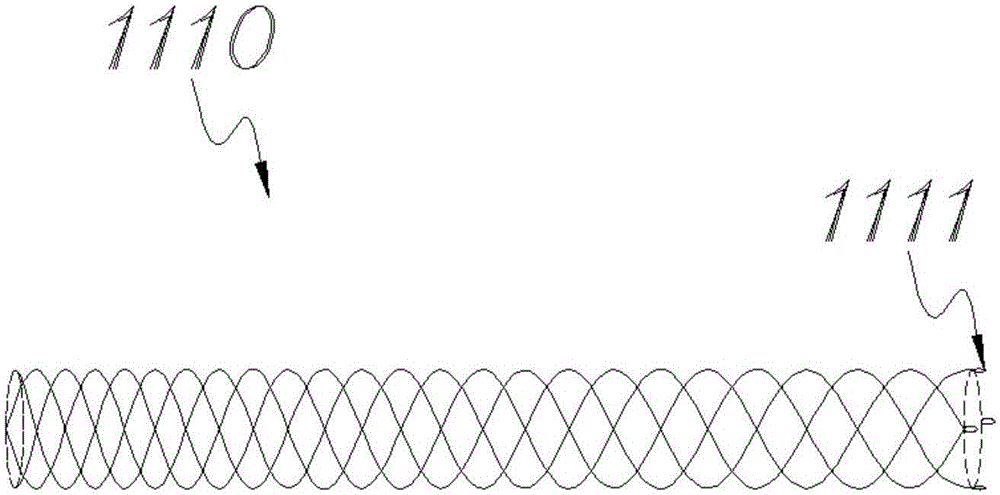
[0039] See image 3 The stent 1110 provided in this embodiment includes a tubular stent body braided by four nickel-titanium wires using the Coil braiding method. Similar to Example 1, the braiding density of the tubular stent body gradually increases from one end to the other. loose, wherein the loose end forms a connecting structure 1111 with four pull rings.
[0040] Dissolve 300 mg of sodium alginate and 20 mg of chitosan in 10 ml of purified water, heat to 50°C, add 0.1 ml of glycerin, stir evenly for 2 hours, and degas under vacuum to obtain a degassed liquid. Coat a layer of Tween 80 on the glass plate, then evenly spray paclitaxel in ethanol solution, and let it stand for 30 minutes. Pour the degassed liquid into the above glass plate, and cast it into a film. Dry the glass plate in an oven at 50 degrees for 4 hours, and remove the film. Promptly obtain the absorbable film 1310 with medicine, see Figure 4 .
[0041] The above film 1310 is electrostatically bonded...
Embodiment 3
[0048] See Figure 10 and Figure 11 The stent main body 350 and the stent connection structure 450 are formed by laser engraving and heat treatment expansion of the nickel-titanium pipe. The stent main body 350 is a mesh structure, and the mesh between the stent body 350 and the stent connection structure 450 is a network with dense and gradually sparse mesh. The wedge-shaped transition structure 360. The wedge-shaped transition structure 360 makes it easier for the stent to be folded and pulled into the catheter.
[0049] See Figure 11 , suture the latex film 100 inside the stent, the stent connection structure 450 and the wedge-shaped transition structure 360 do not sew the film;
[0050] Dissolving 75 mg of paclitaxel and 45 mg of polyurea in 2-4 ml of acetone and ethanol to form a mixed solution, spraying the mixed solution on the surface of the membrane, and drying to obtain the stent graft with the drug layer 200;
[0051] Please continue to see Figure 12 , th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com