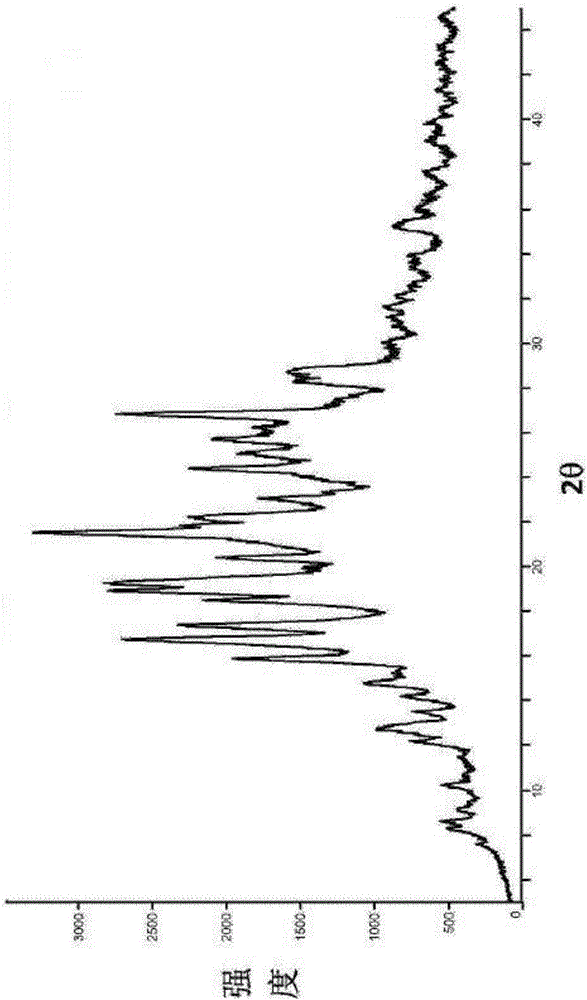
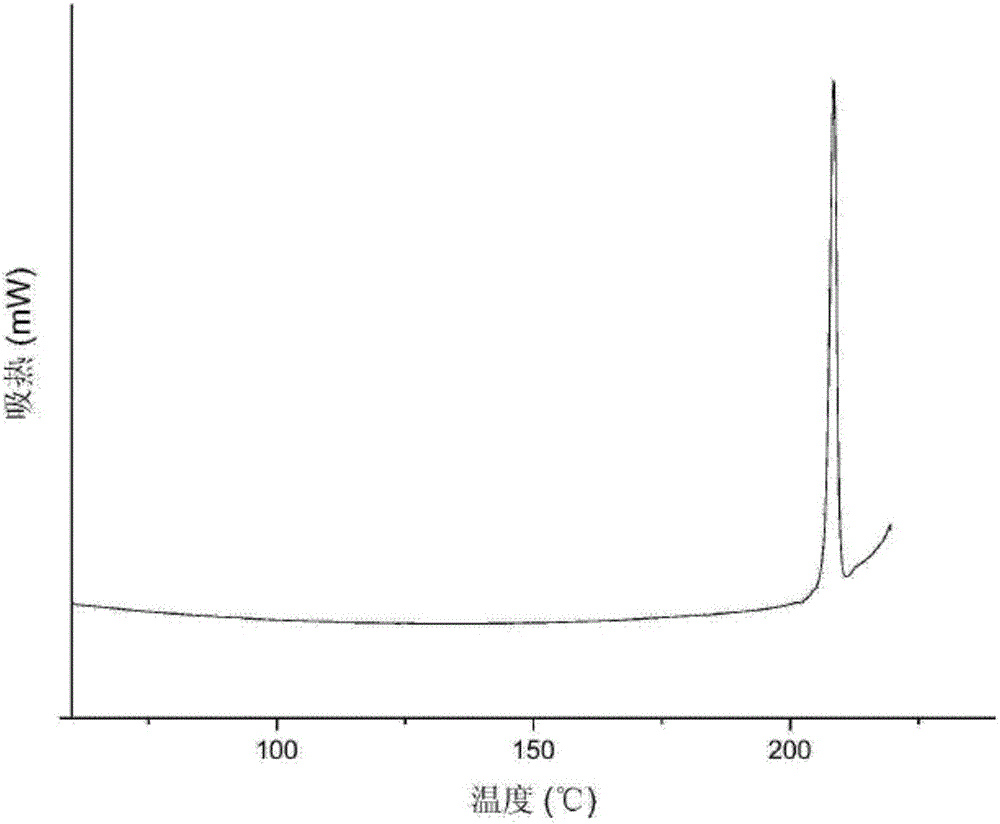
Metformin hydrochloride sustained-release tablet and preparation method thereof
A metformin hydrochloride and sustained-release tablet technology, which is applied in the field of medicine, can solve the problems of narrow absorption range, non-absorption, and decreased bioavailability in the gastrointestinal tract, and achieve easy absorption, low hygroscopicity, and avoidance of burst release. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
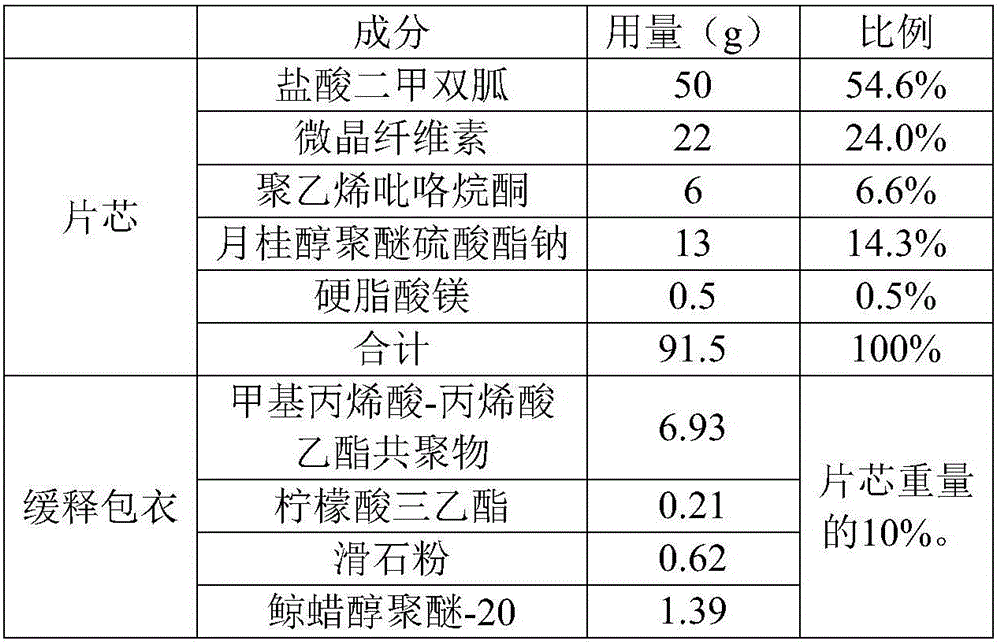
[0031] Prescription, see Table 1:
[0032] Table 1 Example 1 prescription component list
[0033]
[0034] Preparation method:
[0035] (1) Sterilize the reactors, filters, utensils, etc. used. Add 1000g of crude metformin hydrochloride into a 15L three-neck flask, add 8000mL of water, stir, heat to reflux to dissolve, then add 2400mL tetrahydrofuran and 5600mL tert-butanol to the solution, then slowly cool down to 4°C, stir and crystallize, filter, and wash with ether , dried under vacuum at 90° C. to obtain 953 g of metformin hydrochloride as a dry product, with a yield of 95.3%;
[0036] (2) The metformin hydrochloride obtained in step (1) is mixed with a filler and an absorption accelerator for subsequent use, the adhesive is formulated into a 5% aqueous solution, and the prepared adhesive solution is added to the mixed material and mixed uniformly to prepare Soft materials, granulated through a 18-mesh sieve;
[0037] (3) drying the granules obtained in step (2) at 4...
Embodiment 2
[0055] Adjust the prescription according to the ratio in the following table, and prepare the products of Examples 2 to 5 in the same way.
[0056] Table 4 Embodiment 2~5 prescription composition list
[0057]
[0058] The preparation method is the same as in Example 1, only the auxiliary materials are replaced according to the actual situation in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com