Medical dressing and preparation method thereof
A medical, acid-method gelatin technology, applied in medical science, absorbent pads, bandages, etc., can solve the problems of increased toxicity, brittleness, and low mechanical strength of hydrogels in the gel system, achieve good barrier effect, and reduce the incidence rate , the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
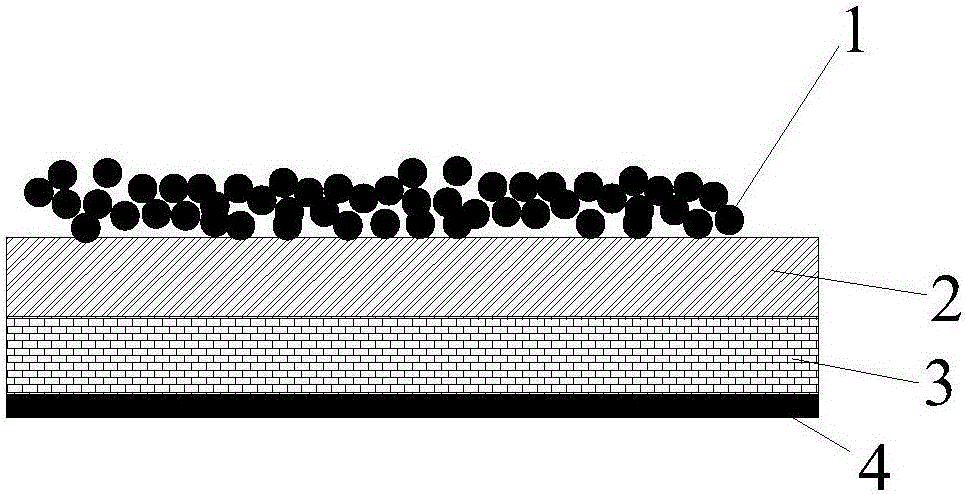
[0040] Such as figure 1 As shown, the medical dressing of the present invention is made of non-woven fabric 3 (preferably medical non-woven fabric), and a waterproof and moisture-permeable layer 2 is sprayed on the front direction of the non-woven fabric 3, and the waterproof and moisture-permeable layer 2 is made of polyurethane At the same time, the pore size in the polyurethane nanofiber membrane is controlled between 0.2 and 1 μm (preferably 0.5 μm), because the pore size is too large, the waterproof ability of the polyurethane nanofiber membrane and the ability to block particles such as bacteria will decline, while If the pore size is too small, it is not conducive to the moisture permeability of the dressing. The pore size of the polyurethane nanofiber membrane is between 0.2 and 1 μm, which ensures a good barrier effect on bacteria and has a good air permeability effect. The surface of the waterproof and moisture-permeable layer 2 is coated with gelatin nanoparticles 1...
Embodiment 2
[0050] The present embodiment provides the preparation method of this medical dressing, and concrete preparation steps are as follows:
[0051] 1) Polyurethane nanofibers are prepared by thermoplastic nanofiber preparation technology, and the average diameter of polyurethane nanofibers is controlled between 200 and 500nm. The average diameter of polyurethane nanofibers prepared in this implementation is 250nm; , the polyurethane nanofiber suspension was obtained, and the mass concentration percentage of the polyurethane nanofiber suspension was controlled at 20 to 35%, and the mass concentration percentage of the polyurethane nanofiber suspension prepared in this embodiment was 21.6%;
[0052] In order to prepare the nanofiber membrane, the polyurethane nanofiber suspension with a mass concentration of 21.6% is evenly sprayed on the front of the medical non-woven fabric, and then dried with hot air to prepare a medical non-woven fabric composited with the polyurethane nanofiber...
Embodiment 3
[0058] The present embodiment provides the preparation method of this medical dressing, and concrete preparation steps are as follows:
[0059] 1) Polyurethane nanofibers are prepared by thermoplastic nanofiber preparation technology, and the average diameter of polyurethane nanofibers is controlled between 200 and 500nm. The average diameter of polyurethane nanofibers prepared in this implementation is 400nm; , the polyurethane nanofiber suspension is obtained, and the mass concentration percentage of the polyurethane nanofiber suspension is controlled at 20 to 35%, and the mass concentration percentage of the polyurethane nanofiber suspension prepared in this implementation is 25.8%;
[0060] In order to prepare the nanofiber membrane, the polyurethane nanofiber suspension with a mass concentration of 25.8% is evenly sprayed on the front of the medical non-woven fabric, and then dried with hot air to prepare a medical non-woven fabric compounded with polyurethane nanofibers. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com