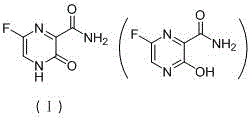
6-fluorine-3-hydroxy-2-pyrazinamide synthetic method
A synthetic method, the technology of pyrazinamide, applied in the direction of organic chemistry, etc., can solve the problems of long synthetic route steps, high activity of fluorine atoms, low total yield of the process, etc., and achieve simple method, short synthetic route and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
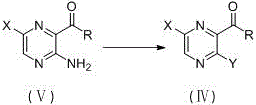
Image
Examples
Embodiment 1
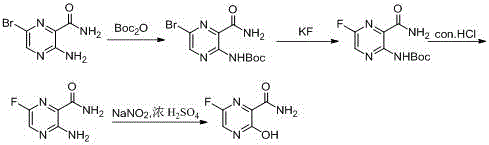
[0055] Embodiment 1: Preparation of 3,6-dibromo-2-pyrazinecarboxylic acid methyl ester
[0056] In 600ml N,N-dimethylformamide, add 120g (0.52mol) methyl 6-bromo-3-aminopyrazinecarboxylate, 181g copper bromide (0.78mol), stir and mix at room temperature, add 90.87 g (0.78mol) of isoamyl nitrite, after the dropwise addition, the temperature was raised to 65°C for 2-3 hours. After completion of the reaction, cool down to room temperature, add 200ml of water and 1L of ethyl acetate for extraction, separate the layers, wash the organic phase with saturated brine and saturated sodium bicarbonate solution successively, dry, decolorize, and distill under reduced pressure to obtain 150.3g of brown oily liquid. The oil was slurried with petroleum ether to obtain 124 g of off-white solid. Yield: 81.5%. MS:295 [M+H]
[0057] The following table compounds were prepared in the same way:
[0058]
Embodiment 2
[0059] Embodiment 2: Preparation of 3,6-dibromo-2-pyrazinamide
[0060] In 850ml of methanol, add 85g (0.29mol) of methyl 3,6-dibromo-2-pyrazinecarboxylate, add 1.7L of ammonia water, and stir at room temperature for 2 to 3 hours. Filter, rinse the filter cake with methanol, and dry to obtain 75 g of light yellow solid. Yield: 92%. MS:280 [M+H]
[0061] The following table compounds were prepared in the same way:
[0062]
Embodiment 3
[0063] Example 3: Preparation method 1 of 3,6-difluoro-2-pyrazinamide
[0064] In 50ml dimethyl sulfoxide, add 5g (17.8mmol) 3,6-dibromo-2-pyrazinamide, 6.2g (106.8mmol) potassium fluoride, 1.2g (3.56mmol) tetrabutylammonium bromide , heated to 150 ° C for 3 h. The reaction was completed, lowered to room temperature, added 50ml of water and 150ml of ethyl acetate for extraction, separated, the organic phase was washed with saturated brine, dried, decolorized, concentrated under reduced pressure to obtain an oily crude product, and recrystallized with isopropanol to obtain 1.2g of Yellow target product. Yield: 43%. MS:160 [M+H]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com