A car-t cytotoxicity indicator carrier
A cytotoxic and cellular technology, applied in the direction of virus/bacteriophage, retroRNA virus, using vectors to introduce foreign genetic material, etc., can solve the research and application development constraints of CAR-T cell therapy technology, long detection time, lack of CAR-T T cell toxicity detection method R&D and application of CAR-T cell therapy technology to achieve excellent production prospects, shortened time, excellent flexibility and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Construction of CAR-T cytotoxicity indicator vector
[0034] (1) Synthesis of CD19-P2A- Luciferase sequence, and introduce Asis I and MluI restriction sites in the upstream and downstream respectively;
[0035] (2) Enzyme digestion of Plent-EF1α-Puro-CMV vector and CD19-P2A containing Asis I and MluI restriction sites obtained in step (1) Luciferase sequence, enzyme digestion system is as follows:
[0036]
[0037]
[0038] After adding and mixing the sample, place it at 37°C for enzyme digestion for 3 hours. After the reaction, use 1% agarose gel electrophoresis to detect the digested CD19-P2A-Nanoluc Luciferase sequence and Plent-EF1α-Puro-CMV vector. And use the DNA gel recovery kit to recover the digested CD19-P2A- Luciferase sequence and Plent-EF1α-Puro-CMV vector;
[0039] (3) CD19-P2A- The Luciferase sequence and the Plent-EF1α-Puro-CMV vector were ligated at 22°C for 2 hours, and the ligation system was as follows,
[0040]
[0041] ...
Embodiment 2
[0042] Embodiment 2 packaging lentivirus
[0043] (1) Transfer HEK293T cells whose cell growth is more than 90% of the maximum load capacity of a 10cm culture dish to a 10cm cell culture dish at a ratio of 1:3 (the number of cells per dish is about 2.5×10 6 ), placed in a 37°C, 5% CO2 incubator for 24 hours; obtain the cells to be transfected; the ratio of 1:3 refers to the cells in one 10cm culture dish, which are averagely passaged to three same culture dishes;
[0044] (2) Change the medium before transfection, and replace 5 ml of DMEM medium containing 10% FBS with the cells to be transfected obtained in step (1) with an electric pipette to obtain cells before transfection;
[0045] Prepare the transfection reagent as follows:
[0046]
[0047]
[0048] Prepare reagents 1 and 2 respectively, and place them at room temperature for 5-10 minutes; mix reagents 1 and 2 evenly, and place them at room temperature for 15-30 minutes to obtain reagent 3; add reagent 3 dropwis...
Embodiment 3
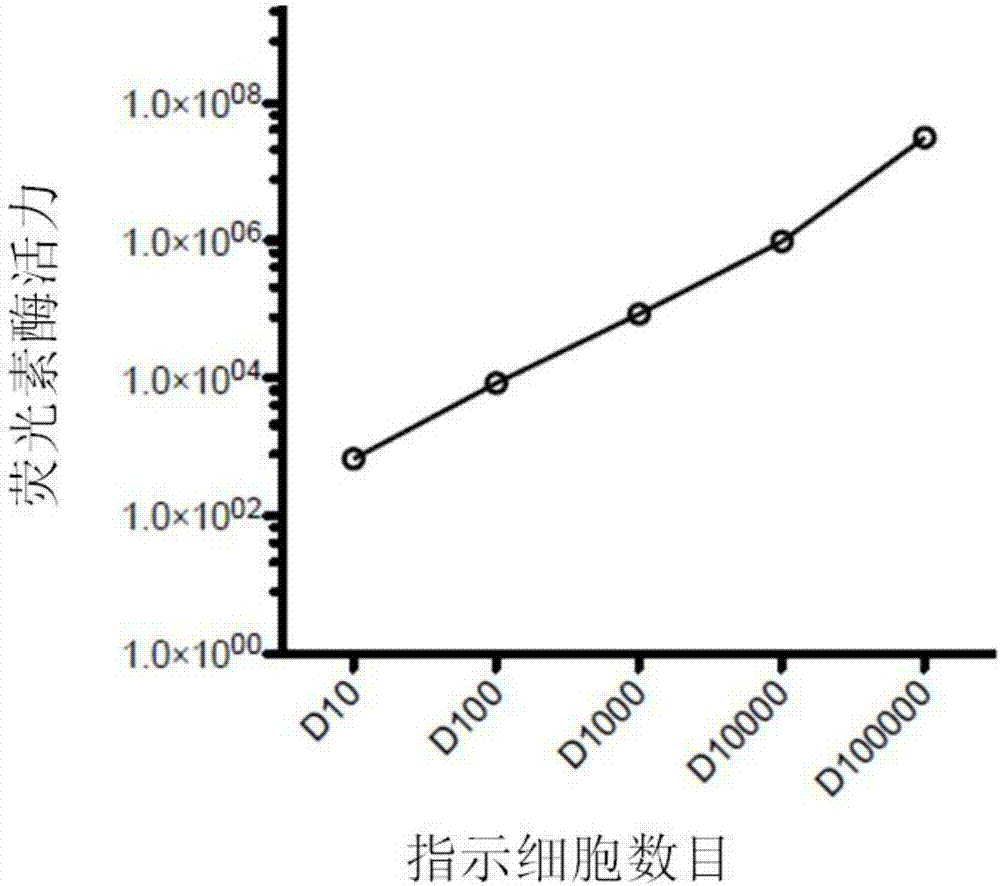
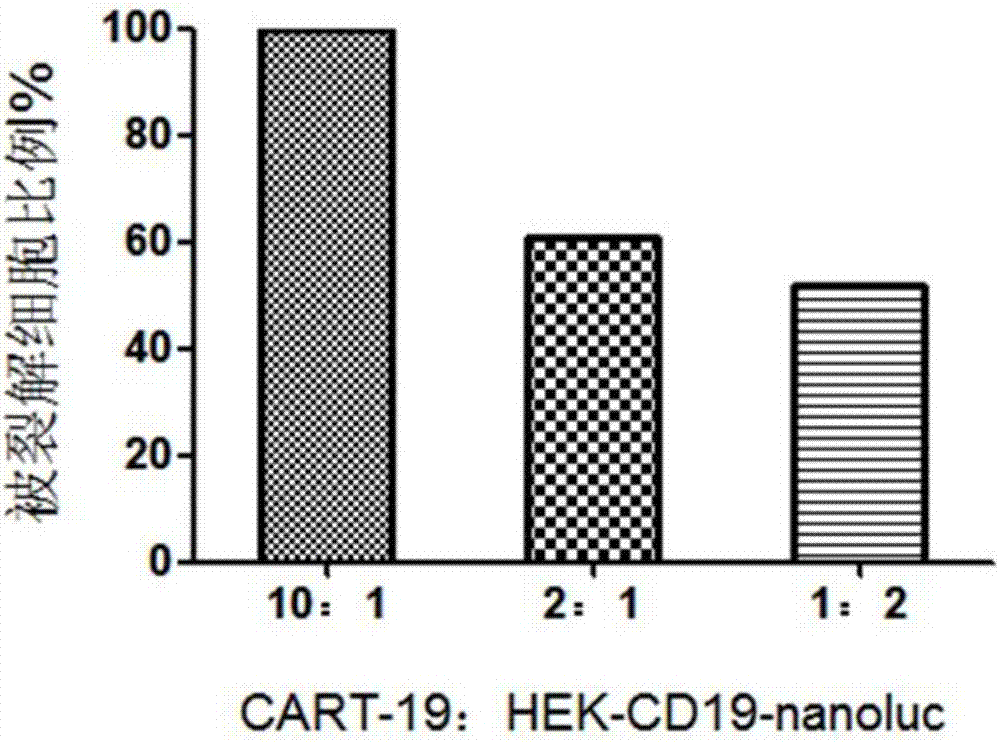
[0049] Example 3 Preparation of CAR-T cytotoxicity indicator cells
[0050] Infection experiments were carried out according to conventional methods known to those skilled in the art, and the infection steps are briefly described as follows:
[0051] (1) Lentivirus infection
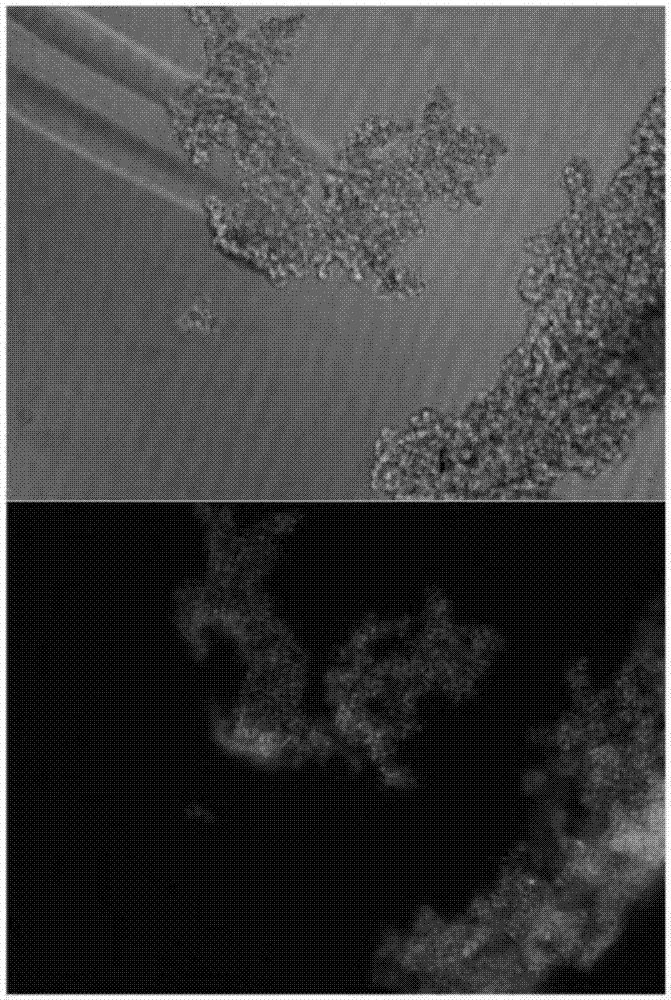
[0052] In a 6-well plate, use the co-infection reagent ADV-HR and the lentiviral particles obtained in Example 2 to infect K562 cells, calculate the amount of lentiviral particles according to the multiplicity of infection (MOI=10), and use DMEM medium every other day during the infection process. Cells were exchanged to maintain the cell concentration at 0.5-2×10 6 / mL.
[0053] (2) Resistance screening
[0054] 72 hours after infection, start resistance screening:
[0055] Puromycin at a concentration of 2 μg / mL was selected for 14 days to obtain polyclonal cell lines with puromycin resistance, and monoclonal cell lines were obtained by further screening with puromycin at a concentration of 2 μg / mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com