A kind of preparation method of tetrafluoroborate spirocyclic ammonium salt
A tetrafluoroborate spiro and spiro quaternary ammonium salt technology, applied in the direction of organic chemistry, can solve the problems of accelerating the capacity decay of supercapacitors, affecting, reducing the initial capacity and cycle life of supercapacitors, and achieving mild reaction conditions and energy consumption. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
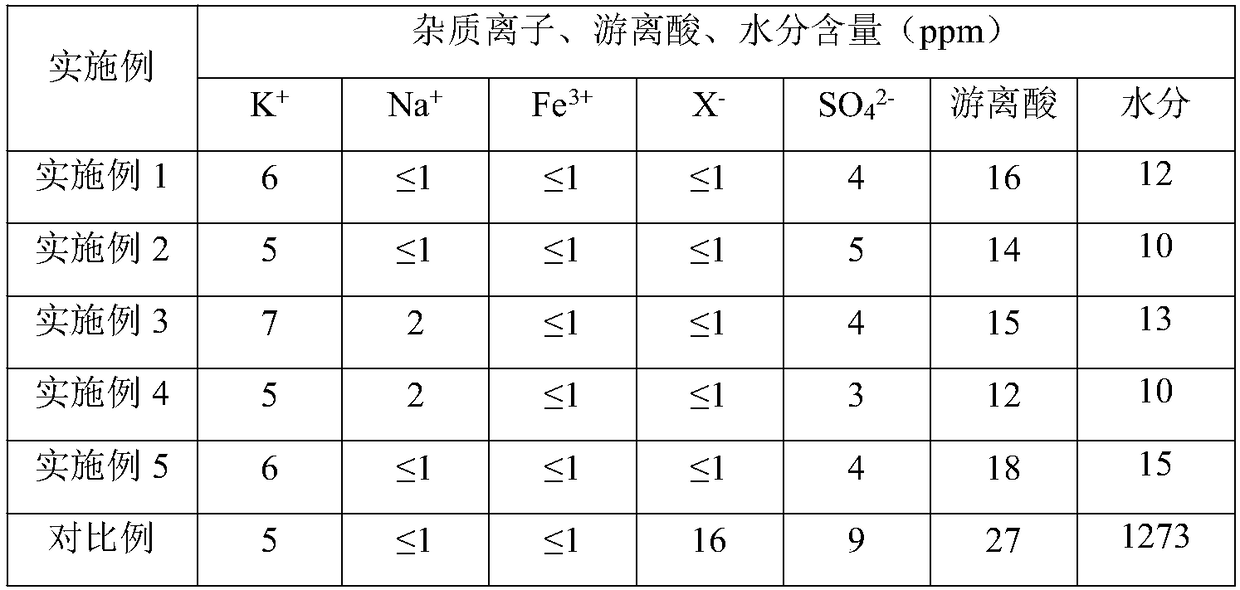
[0028] In the 2000mL reaction kettle 1, 1000mL of acetonitrile solvent, 194.3g of potassium carbonate, 16.8g of potassium bromide were sequentially added under stirring, and 100.0g of tetrahydropyrrole and 178.6g of 1,4-dichlorobutane were slowly added in sequence under nitrogen protection , stirred and refluxed at 85°C for 12h, cooled and filtered to obtain a chlorinated spirocyclic quaternary ammonium salt solution. Add 265.5g of potassium tetrafluoroborate to the chlorospiroquaternary ammonium salt solution, stir at 25°C for 12 hours for ion exchange, filter, add 5.0g of potassium carbonate to the mother liquor, stir, filter, and distill under reduced pressure to obtain spiroquaternary tetrafluoroborate Crude salt. The crude spirocyclic ammonium salt of tetrafluoroborate was extracted three times with ethyl acetate / water with a volume ratio of 1:1, and the water phase was collected; vacuum distillation, removal of water, heating and dissolving with ethanol, stirring at -5°C...
Embodiment 2
[0035]In a 2000mL reaction kettle 1, 1000mL of acetonitrile solvent, 291.5g of potassium carbonate, 22.7g of tetrabutylammonium bromide were successively added under stirring, and 100.0g of tetrahydropyrrole and 214.2g of 1,4 -Dichlorobutane, stirred and refluxed at 80°C for 10h, cooled and filtered to obtain a chlorospirocyclic quaternary ammonium salt solution. Add 221.3g of potassium tetrafluoroborate to the chlorospiroquaternary ammonium salt solution, stir at 25°C for 12 hours for ion exchange, filter, add 3.0g of potassium carbonate to the mother liquor, stir, filter, and distill under reduced pressure to obtain spiroquaternary tetrafluoroborate Crude salt. The crude spirocyclic ammonium salt of tetrafluoroborate was extracted 3 times with ethyl acetate / water with a volume ratio of 1:1, and the water phase was collected; vacuum distillation, removal of water, heating and dissolving with ethanol, stirring at -10°C Recrystallization, the obtained solid was heated and diss...
Embodiment 3
[0038] In a 2000mL reaction kettle 1, 1000mL of acetonitrile solvent, 388.7g of potassium carbonate, 45.3g of tetrabutylammonium bromide were sequentially added under stirring, and 100.0g of tetrahydropyrrole and 214.2g of 1,4 -Dichlorobutane, stirred and refluxed at 75°C for 12h, cooled and filtered to obtain a chlorospirocyclic quaternary ammonium salt solution. Add 221.3g potassium tetrafluoroborate to the chlorospiroquaternary ammonium salt solution, stir at 20°C for 10h for ion exchange, filter, add 6.0g sodium carbonate to the mother liquor, stir, filter, and distill under reduced pressure to obtain spiroquaternary tetrafluoroborate Crude salt. The crude product of spirocyclic quaternary ammonium tetrafluoroborate was extracted three times with ethyl acetate / water at a volume ratio of 1:1, and the aqueous phase was collected; distilled under reduced pressure to remove water, dissolved by heating with ethanol, and stirred again at 0°C. Crystallization, the obtained solid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com