Preparation method of artemisinin molecular imprinting photoelectrochemical sensor
A technology of molecular imprinting and photoelectrochemistry, which is applied in the field of analysis, detection and sensors, can solve the problems of poor selectivity, complicated operation and high cost, and achieve the effect of low cost, simple operation and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
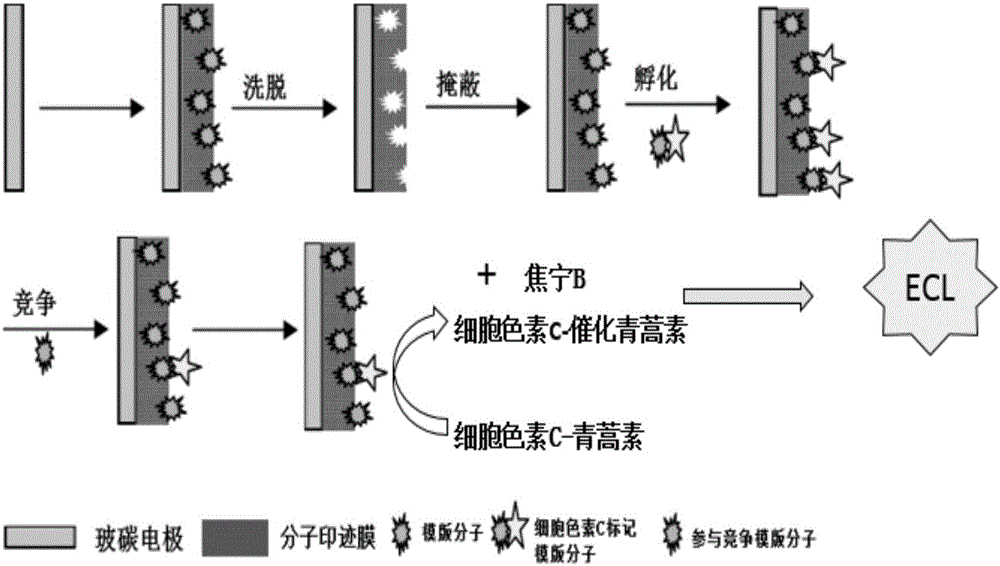
[0028] Polish the glassy carbon electrode with 0.3 μm and 0.05 μm alumina paste, then put it into a mixed solution of distilled water and acetaldehyde for ultrasonic cleaning, take it out and blow it dry with nitrogen. Immerse the treated glassy carbon electrode in a deoxyphosphate buffer solution containing 14 μM artemisinin, 0.075 mM acrylamide, 0.75 mM ethylene glycol dimethacrylate, 0.06 mM azobisisobutyronitrile, and 0.1 M potassium chloride . In the range of -0.2 ~ 1.2V, the scan rate is 100mVs -1 , using cyclic voltammetry to scan for 7 to 10 cycles until the scanning curve is stable. Soak the electrode in a mixed solution of acetic acid and methanol for 1 hour to elute the template molecules to obtain an artemisinin molecularly imprinted electrode. At 25°C, immerse the prepared molecularly imprinted electrode in a solution containing 4 mM artemisinin to cover the cavity for 15 min, then put it in a solution containing cytochrome C-labeled artemisinin for 20 min, and ...
Embodiment 2
[0030] Polish the glassy carbon electrode with 0.3 μm and 0.05 μm alumina paste, then put it into a mixed solution of distilled water and acetaldehyde for ultrasonic cleaning, take it out and blow it dry with nitrogen. Immerse the treated glassy carbon electrode in a deoxyphosphate buffer solution containing 13.6 μM artemisinin, 0.065 mM acrylamide, 0.78 mM ethylene glycol dimethacrylate, 0.056 mM azobisisobutyronitrile, and 0.1 M potassium chloride middle. In the range of -0.2 ~ 1.2V, the scan rate is 100mVs -1 , using cyclic voltammetry to scan for 10 to 15 cycles until the scanning curve is stable. Soak the electrode in a mixed solution of acetic acid and methanol for 1 hour to elute the template molecules to obtain an artemisinin molecularly imprinted electrode. At 25°C, immerse the prepared molecularly imprinted electrode in a solution containing 4 mM artemisinin to cover the cavity for 20 min, then put it in a solution containing cytochrome C-labeled artemisinin for 30...
Embodiment 3
[0032]Polish the glassy carbon electrode with 0.3 μm and 0.05 μm alumina paste, then put it into a mixed solution of distilled water and acetaldehyde for ultrasonic cleaning, take it out and blow it dry with nitrogen. Immerse the treated glassy carbon electrode in a deoxyphosphate buffer solution containing 14 μM artemisinin, 0.07 mM acrylamide, 0.76 mM ethylene glycol dimethacrylate, 0.055 mM azobisisobutyronitrile, and 0.1 M potassium chloride . In the range of -0.2 ~ 1.2V, the scan rate is 100mVs -1 , using cyclic voltammetry to scan for 15 to 20 cycles until the scanning curve is stable. Soak the electrode in a mixed solution of acetic acid and methanol for 1 hour to elute the template molecules to obtain an artemisinin molecularly imprinted electrode. At 25°C, the prepared molecularly imprinted electrode was immersed in a solution containing 5.5 mM artemisinin to cover the cavity for 15 min, then it was placed in a solution containing cytochrome C-labeled artemisinin fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com