Macrolide derivatives and application thereof
A technology of macrolides and derivatives, which is applied in the field of macrolides derivatives, can solve the problems of drug development and use limited by antibiotic activity, achieve enhanced gastrointestinal motility, low antibiotic activity, and promote digestive tract dynamic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
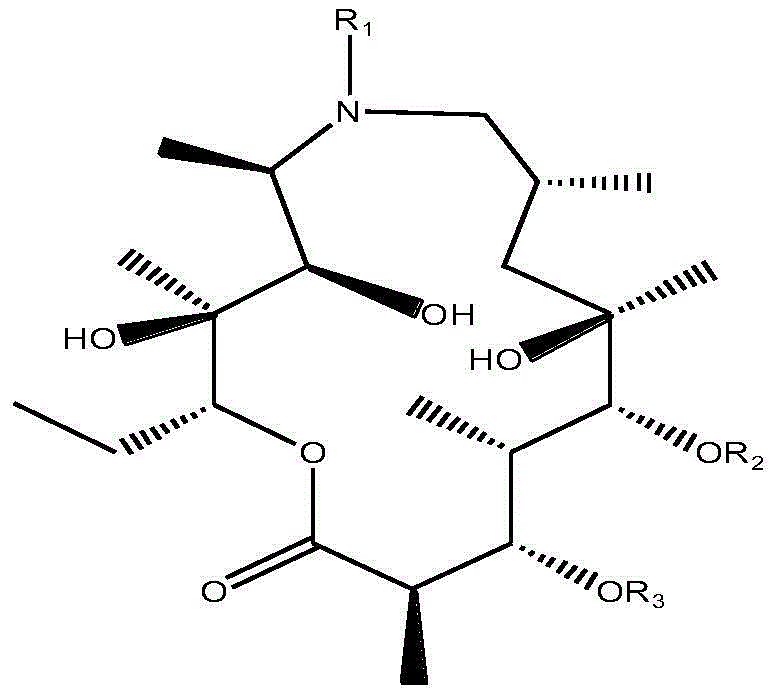
[0020] The present invention prepares compound macrolide derivatives through chemical reaction, by 1 H-NMR (proton nuclear magnetic resonance spectrum) and MS (mass spectrometry) carried out structure identification on macrolide derivatives. The antibacterial activity and the activity of promoting intestinal motility of the macrolide derivatives were detected through a series of experiments. The specific implementation is as follows:
[0021] In the following examples, used test materials and sources thereof include:
[0022] (1) mouse
[0023] Kunming mice (female): provided by the Experimental Animal Center of the Academy of Military Medical Sciences of the Chinese People's Liberation Army and Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.
[0024] After the animals arrive, special personnel will receive the animals in the double-corridor barrier environment mouse breeding room, fill in the "Test Animal Reception Record Form" (BG-017-V00), observe the gene...
Embodiment 1
[0037] Synthesis and identification of embodiment 1 macrolide derivatives
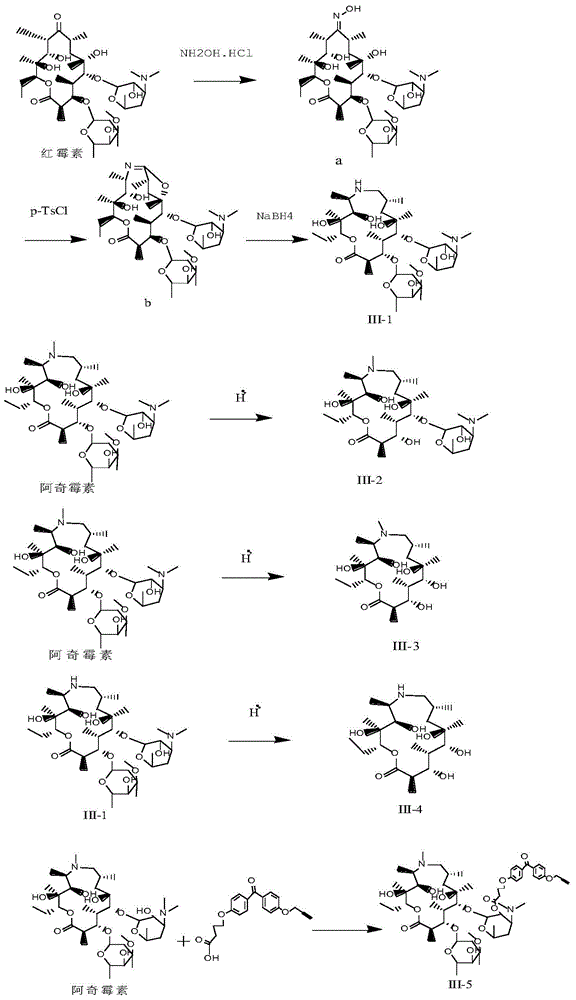
[0038] Dissolve 28.58g (38.94mmol) of erythromycin A and 15.6g (224.4mmol) of hydroxylamine hydrochloride in 60.0ml of anhydrous methanol, then add 15.6ml (112.4mmol) of triethylamine, stir and heat under reflux for 24 hours, after the reaction is completed , cooling and suction filtration at 0°C, washing the filter cake with a small amount of methanol, suspending the resulting solid in 80ml of methanol, adding 20ml of ammonia water dropwise under stirring, filtering to remove insoluble substances, adding the methanol ammonia solution dropwise into 150ml of water, a large amount of white solids precipitated , filtered with suction, washed with water until neutral, and dried to obtain solid a (see figure 1 ), the productive rate is 80.6%, and the melting point is 163-166° C., and the mass spectrometry data is MS (M / Z): 749.75 (M+H + ).
[0039] Dissolve 0.1 g (0.134 mmol) of 9-oxime erythromycin A (a)...
Embodiment 2
[0045] Antibacterial activity test of embodiment 2 macrolide derivatives
[0046] experimental method:
[0047]1) Take 5 μL of Escherichia coli glycerol, inoculate into 5 mL of liquid LB medium, place in a shaker at 37°C, 220 rpm / min, and culture overnight;
[0048] 2) Pour the Escherichia coli cultured overnight into 250ml of LB medium, and culture at 37°C and 220rpm for 3 hours;
[0049] 3) After 3 hours, divide the bacterial solution in the Erlenmeyer flask into small test tubes, 5ml in each test tube, add 5μl of the same molar amount of derivatives to each test tube, incubate for 24 hours, test every 2-4 hours OD 600 Value, and calculate the bacteriostatic rate, bacteriostatic rate=(1-(OD of derivative 600 value) / (OD of DMSO blank group 600 value)) x 100%.
[0050] Antibacterial activity test results:
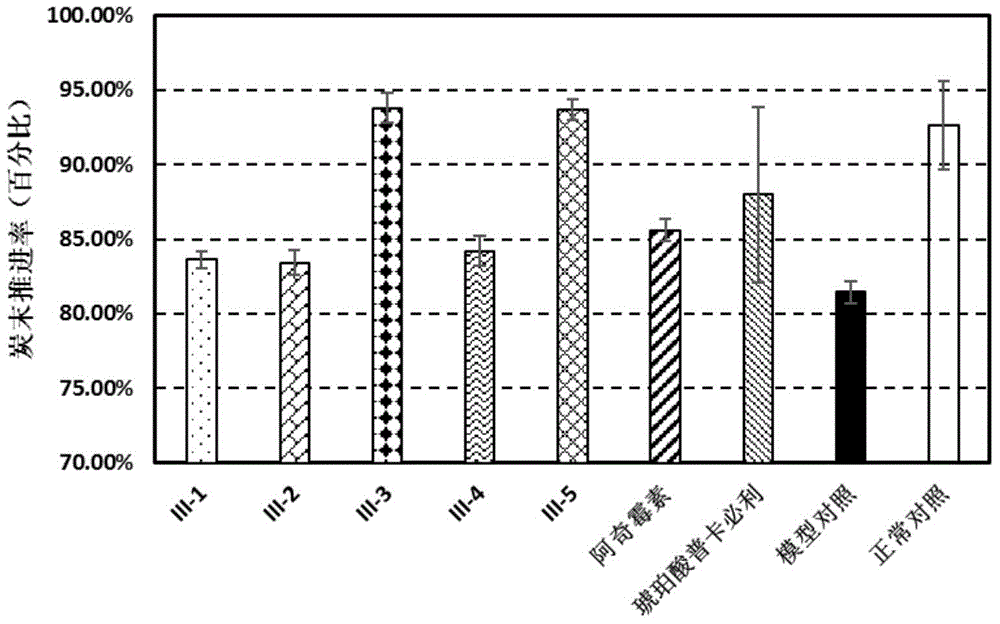
[0051] The bacteriostatic activity of macrolide derivatives is shown in Table 1 and Table 2, wherein Table 1 is the bacteriostatic rate under the concentration of 5.3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com