Atorvastatin calcium tablet and preparation method thereof
A technology of atorvastatin calcium and calcium carbonate, applied in the field of medicine, can solve the problems of poor water solubility, easy degradation, and limited formulation development.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1 The preparation of atorvastatin calcium tablet of the present invention
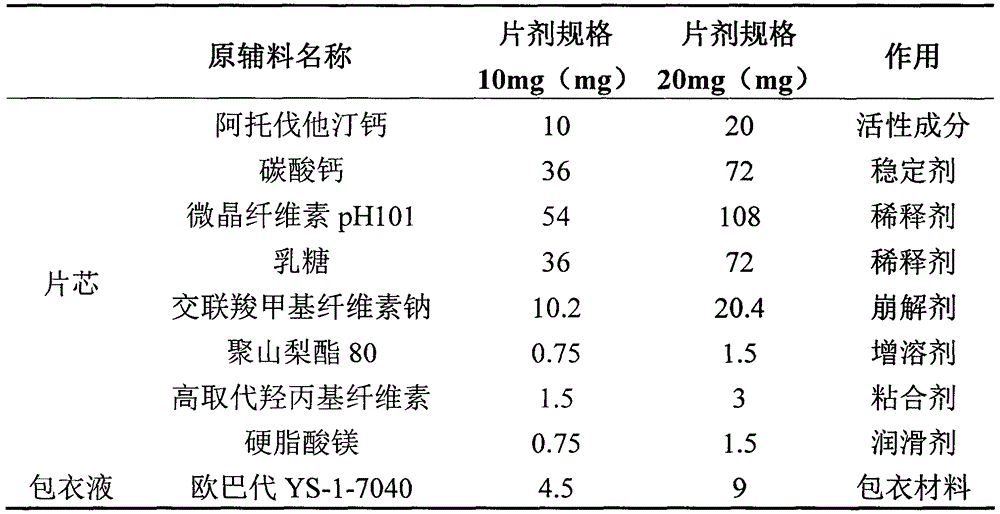
[0018] Prescription Components and Functions of Atorvastatin Calcium Tablets
[0019]
[0020] Preparation Process:
[0021] 1. Take each raw and auxiliary material according to the prescription, and pass through a 100-mesh sieve;
[0022] 2. Preparation of adhesive: prepare an adhesive solution containing 2.4% w / w of Tween-80 and 3.0% w / w of highly substituted hydroxypropyl cellulose (viscosity range 6.0-10.0mpa.s);
[0023] 3. Mixing of raw and auxiliary materials: put microcrystalline cellulose pH101, atorvastatin calcium, internally added croscarmellose sodium, calcium carbonate, and lactose into the wet mixing granulator in sequence, and mix well;
[0024] 4. Granulation: put in binder solution, prepare soft material, and pass the soft material through a 20-mesh sieve;
[0025] 5. Drying: Put the wet granules in an oven and dry at 40°C to 60°C;
[0026] 6. Final blending o...
experiment example 1
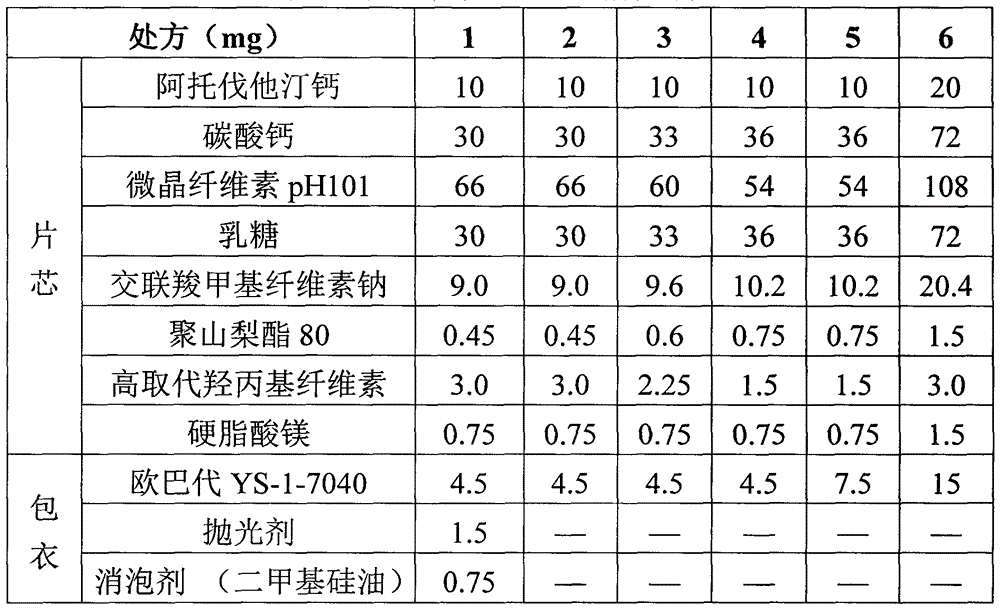
[0030] Experimental Example 1 Atorvastatin Calcium Tablets Prepared According to Different Amounts of Raw Materials
[0031] Table 1 Prescription of Atorvastatin Calcium Tablets
[0032]
[0033] Prepare the tablets of prescriptions 1 to 6 according to the following process:
[0034] The above-mentioned prescription sieves atorvastatin calcium and other auxiliary materials for standby;
[0035] Precisely weigh the raw and auxiliary materials of each prescription, first mix the auxiliary materials except the main drug, and then deliver them in equal amounts.
[0036] Addition method mixes a small amount of the main ingredient and the premixed auxiliary materials evenly;
[0037] Put the mixed material in an appropriate container, add Tween-80-HPC solution, and granulate;
[0038] The wet granules were dried in a blast drying oven at 50°C;
[0039] After the dry granules are sized, an appropriate amount of croscarmellose sodium is added, mixed evenly, and magnesium steara...
experiment example 2
[0049] Experimental Example 2 Preparation of Atorvastatin Calcium Tablets Using Different Process Conditions
[0050] Atorvastatin calcium tablets were prepared according to the prescription and process of Example 1, and the two key process parameters of raw material micronization and granule drying temperature were investigated.
[0051] Table 3 Comparison of process conditions
[0052]
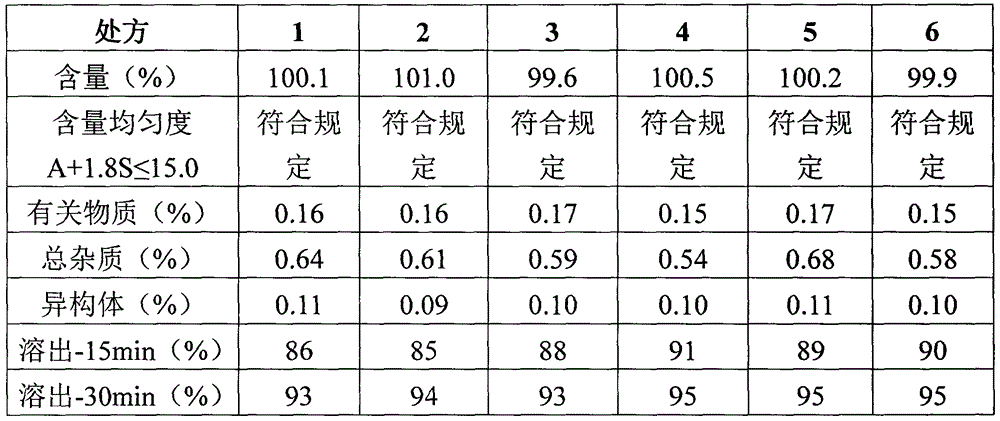
[0053] Table 4 Comparison of quality test results of atorvastatin calcium tablets prepared under different process conditions
[0054]
[0055] After micronizing atorvastatin calcium, its particle size distribution is as follows: D(v, 0.10) 0.355μm, D(v, 0.50) 2.014μm, D(v, 0.90) 11.147μm, that is, the atorvastatin calcium used in this project The particle size of statin calcium raw materials is about 1300 mesh. Comparing prescriptions 7 and 8: the micronization of raw materials is beneficial to the dissolution of the drug (atorvastatin calcium, which belongs to BCS class II).
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com